W424601
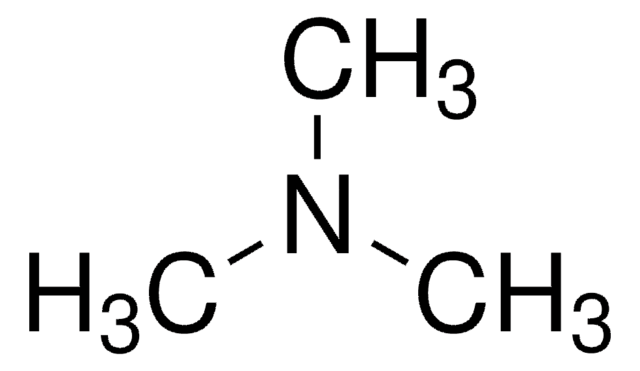
Triethylamine
≥99.5%
Synonym(s):
N,N-Diethylethanamine
About This Item
Recommended Products
biological source
synthetic
Quality Level
reg. compliance
FDA 21 CFR 117
vapor density
3.5 (vs air)
vapor pressure
51.75 mmHg ( 20 °C)
Assay
≥99.5%
form
liquid
autoignition temp.
593 °F
shelf life
5 yr
expl. lim.
8 %
refractive index
n20/D 1.401 (lit.)
pH
12.7 (15 °C, 100 g/L)
bp
88.8 °C (lit.)
mp
−115 °C (lit.)
density
0.726 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
Organoleptic
fishy
SMILES string
CCN(CC)CC
InChI
1S/C6H15N/c1-4-7(5-2)6-3/h4-6H2,1-3H3
InChI key
ZMANZCXQSJIPKH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Novel hybrid thiazoles, bis-thiazoles linked to azo-sulfamethoxazole: Synthesis, docking, and antimicrobial activity.: This study showcases the synthesis of hybrid thiazoles linked to azo-sulfamethoxazole, employing triethylamine in the reaction process, highlighting its role in antimicrobial applications (Salem et al., 2024).
- Spirocyclic rhodamine B benzoisothiazole derivative: a multi-stimuli fluorescent switch manifesting ethanol-responsiveness, photo responsiveness, and acidochromism.: This research demonstrates a spirocyclic rhodamine derivative acting as a multi-stimuli-responsive fluorescent switch, where triethylamine plays a critical role in the synthesis process (Battula et al., 2023).
- Para-Substituted Thiosemicarbazones as Cholinesterase Inhibitors: Synthesis, In Vitro Biological Evaluation, and In Silico Study.: This paper reports on the synthesis of para-substituted thiosemicarbazones where triethylamine is employed as a base, evaluating their potential as cholinesterase inhibitors (Khan et al., 2023).
- Separation and purification of quinolyridine alkaloids from seeds of Thermopsis lanceolata R. Br. by conventional and pH-zone-refining counter-current chromatography.: This study involves the use of triethylamine in the pH-zone-refining counter-current chromatography technique to separate and purify specific alkaloids, illustrating its utility in advanced separation methodologies (Ning et al., 2023).
Disclaimer
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1A - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
12.2 °F - closed cup
Flash Point(C)
-11 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service