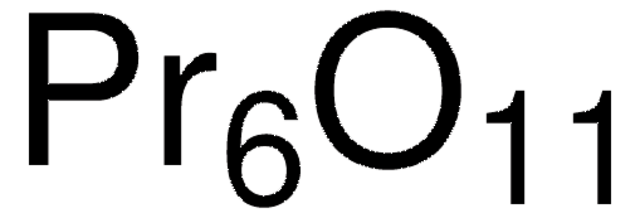
About This Item
Recommended Products
Agency
meets analytical specifications of BP
meets analytical specifications of E252
meets analytical specifications of Ph. Eur.
meets analytical specifications of USP
Quality Level
reg. compliance
meets analytical specifications of FCC
Assay
≥99.9% trace metals basis
≥99.9%
form
powder
reaction suitability
reagent type: catalyst
core: lanthanum
technique(s)
AAS: suitable
density
6.51 g/mL at 25 °C (lit.)
SMILES string
O=[La]O[La]=O
InChI
1S/2La.3O
InChI key
KTUFCUMIWABKDW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Rechargeable solid-state batteries are becoming increasingly important due to wide-spread use in computers, portable electronics, and vehicular applications.
Protocols
This application demonstrates the suitability of the Petrocol column for the efficient analysis of hydrocarbons as listed in ASTM method D5134.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service