906190
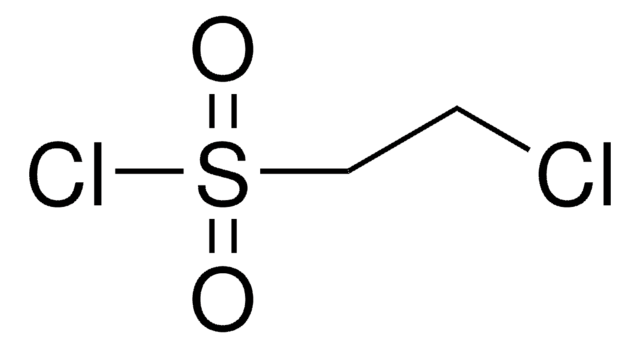
1,2-Dibromoethane-1-sulfonyl fluoride
Synonym(s):
DESF, SuFEx hub, SuFEx-able plugin
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C2H3Br2FO2S
CAS Number:
Molecular Weight:
269.92
MDL number:
UNSPSC Code:
12352101
NACRES:
NA.22
Recommended Products
form
liquid
reaction suitability
reaction type: click chemistry
Application
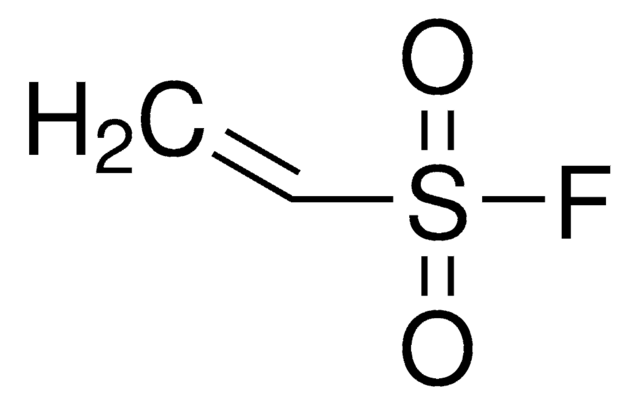
1,2-Dibromoethane-1-sulfonyl fluoride (DESF) is a bench-stable precursor to 1-bromoethene-1-sulfonyl fluoride (BESF), a new and robust connective hub for the Sulfur (VI) fluoride exchange (SuFEx) click reaction. BESF offers similar routes as ethenesulfonyl fluoride (ESF, cat# 746959) but with additional reactivity due to the embedded bromo group.
Treatment of DESF with triethylamine generates BESF in situ, which has been used to synthesize diverse and unprecented sulfonyl fluorides in good-to-excellent yields. As the resulting molecules possess sulfonyl fluoride handles, further SuFEx reactions are also possible. DESF adds another useful tool for conjugation strategies in chemical biology and organic and polymer synthesis.
Automate your fluorination reactions with Synple Automated Synthesis Platform (SYNPLE-SC002)
Treatment of DESF with triethylamine generates BESF in situ, which has been used to synthesize diverse and unprecented sulfonyl fluorides in good-to-excellent yields. As the resulting molecules possess sulfonyl fluoride handles, further SuFEx reactions are also possible. DESF adds another useful tool for conjugation strategies in chemical biology and organic and polymer synthesis.
Automate your fluorination reactions with Synple Automated Synthesis Platform (SYNPLE-SC002)
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jing Leng et al.
Chemical communications (Cambridge, England), 54(35), 4477-4480 (2018-04-17)
A new fluorosulfonylation reagent 1-bromoethene-1-sulfonyl fluoride was developed (1-Br-ESF). This unique reagent possesses three addressable handles (vinyl, bromide, and sulfonyl fluoride) and has great potential to function as a tris-electrophile and as a sulfur(vi) fluoride exchange (SuFEx) clickable material to
Joice Thomas et al.
Organic letters, 20(13), 3749-3752 (2018-06-16)
A regioselective metal-free preparation of 4-fluorosulfonyl 1,2,3-triazoles from organic azides and a hitherto underexplored bromovinylsulfonyl fluoride building block is described. This reaction is very general and was extended to the synthesis of various sulfonates, sulfonamides, and sulfonic acid derivatives of
Christopher J Smedley et al.
Chemical communications (Cambridge, England), 54(47), 6020-6023 (2018-05-26)
We demonstrate 1,2-dibromoethane-1-sulfonyl fluoride (DESF) as a bench-stable and readily accessible precursor to the robust SuFEx connector, 1-bromoethene-1-sulfonyl fluoride (BESF). The in situ generation of BESF from DESF opens up several new reaction profiles, including application in the syntheses of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service