903132
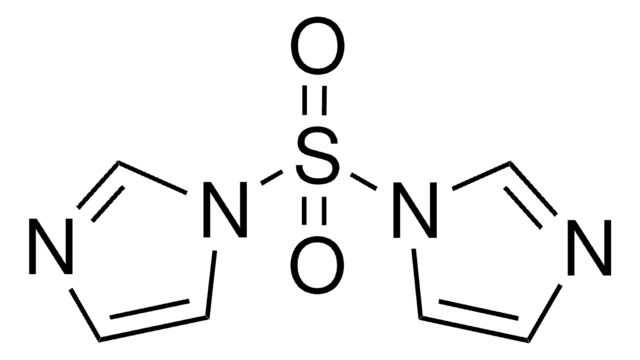
1-(Fluorosulfonyl)-2,3-dimethyl-1H-imidazol-3-ium trifluoromethanesulfonate
≥95%
Synonym(s):
1-(Fluorosulfuryl)-2,3-dimethyl-1H-imidazol-3-ium trifluoromethanesulfonate, Fluorosulfuryl imidazolium triflate salt, SuFEx-IT
About This Item
Recommended Products
Assay
≥95%
form
solid
mp
64-65 °C
storage temp.
2-8°C
Application
Store in dry environment (desiccator advised) at 4 °C.
Automate your fluorination reactions with Synple Automated Synthesis Platform (SYNPLE-SC002)
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
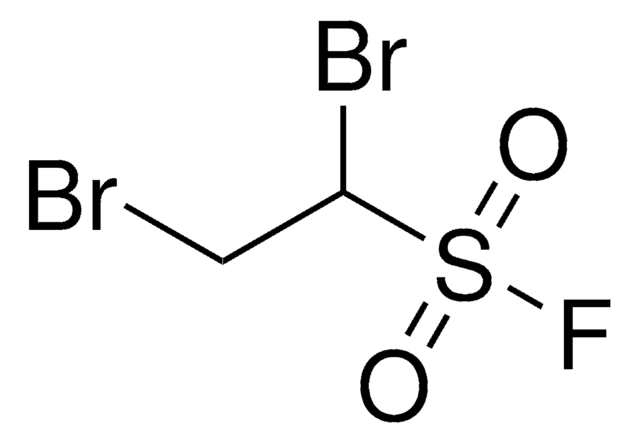
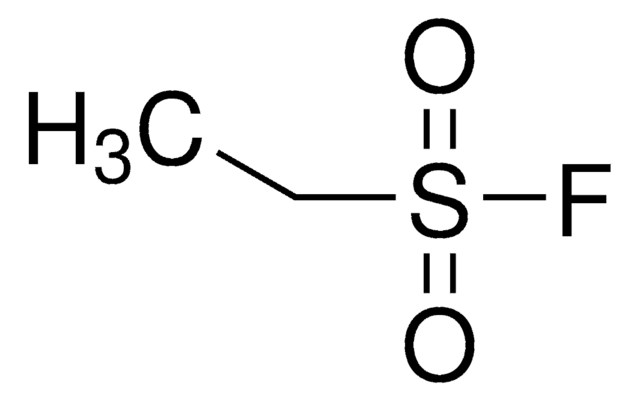
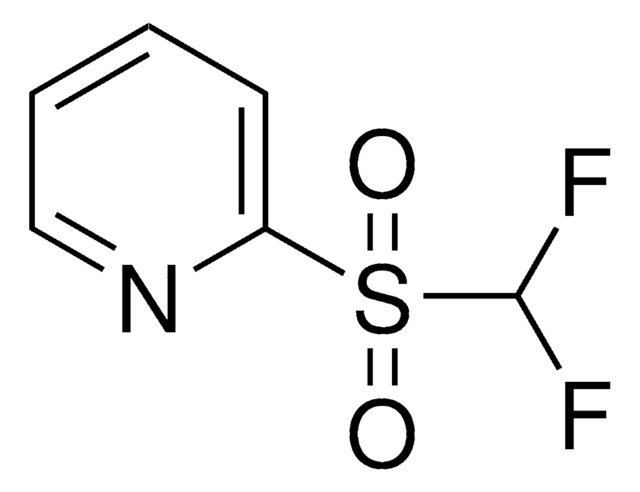
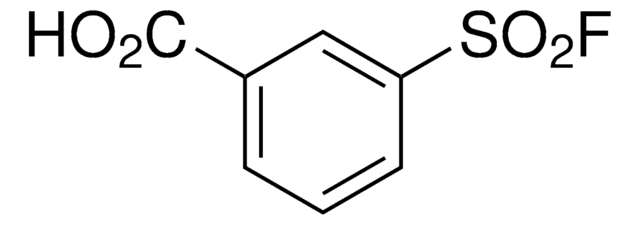
Related Content
The Dong Lab focuses on the synthesis and application of main-group fluorides by studying the reactivity and transformation of those main-group fluorides into organic molecules, including new applications of sulfuryl fluoride chemistry; and new catalysts and process improvements for polysulfate and polysulfonate.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![4-(Acetylamino)phenyl]imidodisulfuryl difluoride ≥98%](/deepweb/assets/sigmaaldrich/product/structures/101/806/3f40354f-e903-4ea0-9654-10872377816c/640/3f40354f-e903-4ea0-9654-10872377816c.png)

![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)