88460
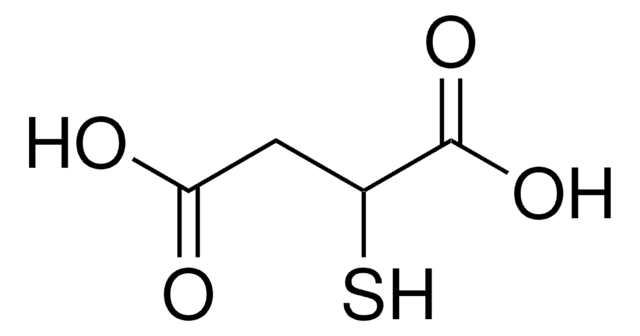
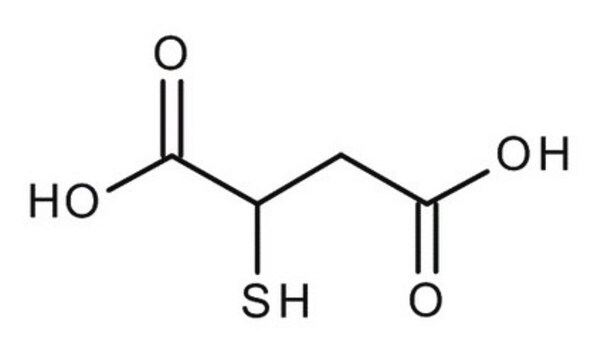
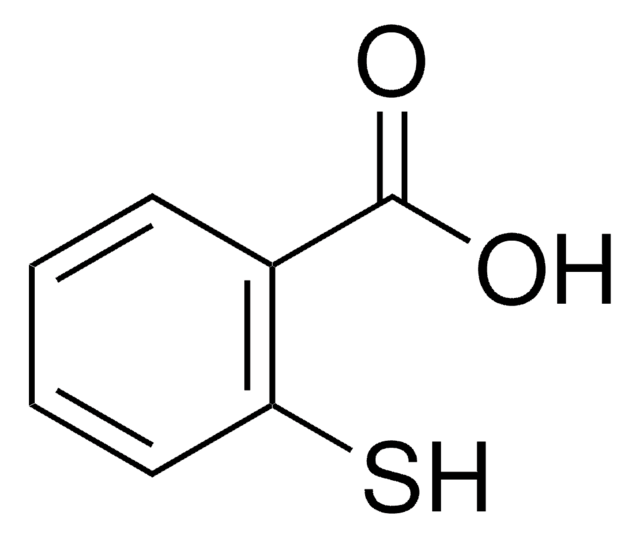
Mercaptosuccinic acid
ReagentPlus®, ≥99.0% (HPLC)
Synonym(s):
Thiomalic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOOCCH(SH)CH2COOH
CAS Number:
Molecular Weight:
150.15
Beilstein:
1099858
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
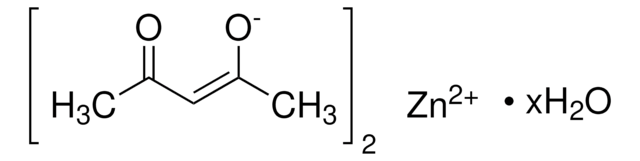
Recommended Products
product line
ReagentPlus®
Assay
≥99.0% (HPLC)
form
powder
mp
145-154 °C
155-157 °C (lit.)
functional group
carboxylic acid
SMILES string
OC(=O)CC(S)C(O)=O
InChI
1S/C4H6O4S/c5-3(6)1-2(9)4(7)8/h2,9H,1H2,(H,5,6)(H,7,8)
InChI key
NJRXVEJTAYWCQJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Mercaptosuccinic acid is a hydrophilic thiol that can form thiomalate self-assembled monolayers when adsorbed on Au(III) surface.
Application
Mercaptosuccinic acid has been used as a dopant for the synthesis of carbon nanodots from citric acid. It can be used as a capping and reducing agent to synthesize monolayer-capped gold nanoparticles.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Surface Chemistry of Thiomalic Acid Adsorption on Planar Gold and Gold Nanoparticles.
Azca?rate J, et al.
Langmuir, 30(7), 1820-1826 (2014)
A switchable peroxidase mimic derived from the reversible co-assembly of cytochrome c and carbon dots.
Essner J, et al.
Journal of Materials Chemistry, 4(12), 2163-2170 (2016)
Jia Zhang et al.
The Analyst, 136(19), 3865-3868 (2011-08-11)
A colorimetric probe based on 2-mercaptosuccinic acid-functionalized gold nanoparticles has been developed to exhibit selectivity towards Ca(2+), Sr(2+), and Ba(2+) ions over other metallic cations under specified conditions and finds its practical application in detecting Ca(2+) levels in water.
Achala de Mel et al.
Regenerative medicine, 7(3), 335-347 (2012-05-19)
This study aimed to live monitor the degree of endothelial progenitor cell (EPC) integration onto tissue-engineering scaffolds by conjugating relevant antibodies to quantum dots (QDs). Biocompatible mercaptosuccinic acid-coated QDs were functionalized with two different antibodies to EPC (CD133 with QDs
Shiho Ishihara et al.
Acta biomaterialia, 6(3), 830-835 (2009-10-20)
Loading and releasing protein in a controllable way is extremely important for the protein vehicles used in bone tissue engineering. To obtain a suitable carrier material for basic proteins, such as BMP or bFGF, hydroxyapatite particles containing mercaptosuccinic acid (mercaptosuccinic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service