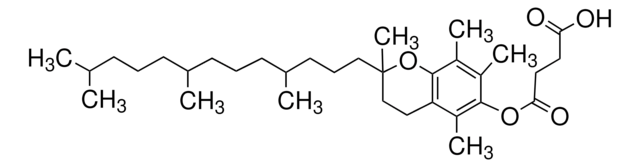
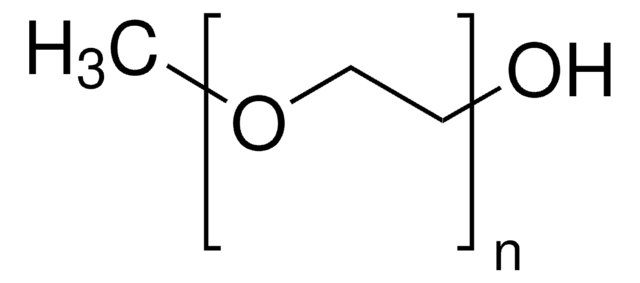
763896
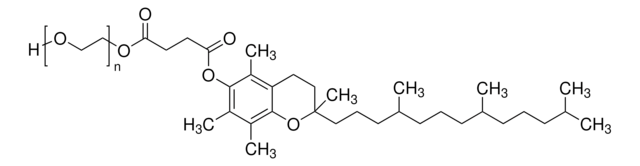
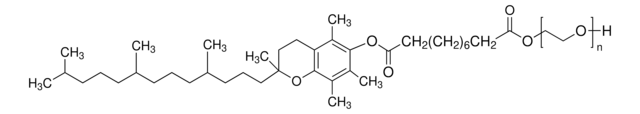
DL-α-Tocopherol methoxypolyethylene glycol succinate
Synonym(s):
Polyethylene glycol, TPGS-750-M
About This Item
Recommended Products
form
powder
Quality Level
reaction suitability
reagent type: catalyst
reaction type: C-H Activation
reagent type: surfactant
greener alternative product characteristics
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
transition temp
Tm 29-34
greener alternative category
, Aligned
General description
Application
TPGS-750-M can also be employed in:
- The nucleophilic aromatic substitution reaction of aryl and heteroaryl halides with nitrogen, oxygen, and sulfur nucleophiles under mild conditions.
- The preparation of quinoxaline-2,3 diones from quinoxalinones via C(sp2)-H hydroxylation reaction.
- The intramolecular N-arylation of amines using a copper catalyst.
Other Notes
From milligrams to kilograms: synthetic chemistry following nature′s lead
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Lipshutz and co-workers have recently developed a second generation technology to their original PTS-enabling surfactant based on the polyoxyethanyl-α-tocopheryl succinate derivative, TPGS-750-M.
Micellular catalysis has provided the ability to carry out several commonly used transformations used in the synthetic community to be carried out in water.
TPGS-750-M, a second generation surfactant, is useful for room temperature, palladium and ruthenium-catalyzed reactions in water. Reactions include the Heck reaction, Suzuki-Miyaura reaction, Sonogashira reaction, Buchwald-Hartwig amination reaction, Negishi reaction, and olefin metathesis.
Protocols
TPGS-750-M, a second generation surfactant, may be used for Suzuki-Miyaura Reactions in Water at Room Temperature
TPGS-750-M, a second generation surfactant, may be used in the Buchwald-Hartwig Amination Reaction in Water at Room Temperature.
Related Content
Prof. Bruce Lipshutz and co-workers have developed designer surfactants to allow several classes of transformations (e.g. Suzuki-Miyaura, Olefin Metathesis, 1,4-Addition to Enones, etc.) to be performed in water.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service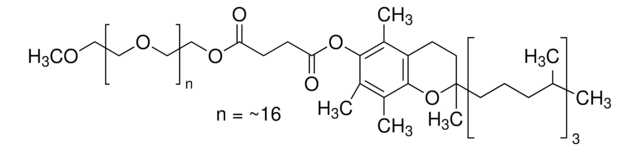
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)


![[1,1′-Bis(di-tert-butylphosphino)ferrocene]dichloropalladium(II) 98%](/deepweb/assets/sigmaaldrich/product/structures/192/459/02d1239c-1119-49d9-b392-a04d8f53855c/640/02d1239c-1119-49d9-b392-a04d8f53855c.png)