655422
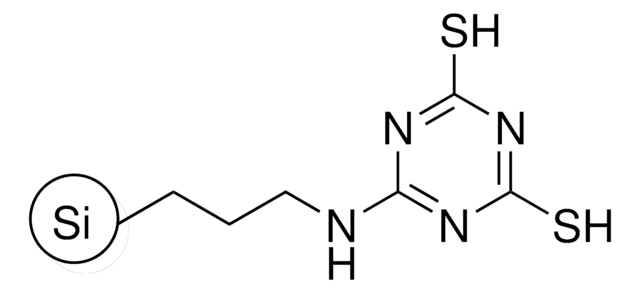
QuadraPure® TU
macroporous, 400-600 μm particle size
Synonym(s):
QuadraPure® Thiourea
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
Quality Level
reaction suitability
reagent type: catalyst
reagent type: chelator
particle size
400-600 μm
General description
QuadraPure® TU is a thiourea-based metal scavenger resin that can be used to prevent metal contamination that occurs during pharmaceutical or fine chemical processing.
Application
Metal Scavenger: Pd, Pt, Ru, Rh, Au, Ag, Cu, Hg, Pb, Cd, Ni, Co, Fe, V, Zn
QuadraPure® TU has been used to remove color from the solution due to leaching of copper species during the copper(I)-mediated 1,2,3-triazole formation via [3+2] cycloaddition of acetylenic compounds with azides. It has also been used as a modifier for carbon pastes to develop QuadraPure® TU residue functionalized resin-modified carbon paste electrode (“TUR-CPE”) for the determination of Pb(II) ions.
Other applications include the removal of metal ions like copper and palladium that leach out during continuous flow coupling reactions.
Other applications include the removal of metal ions like copper and palladium that leach out during continuous flow coupling reactions.
Legal Information
QuadraPure is a registered trademark of Johnson Matthey Finland Oy
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
[3+ 2] Cycloaddition of acetylenes with azides to give 1, 4-disubstituted 1, 2, 3-triazoles in a modular flow reactor.
Smith CD, et al.
Organic & Biomolecular Chemistry, 5(10), 1559-1561 (2007)
Nikzad Nikbin, et al.
Organic Process Research & Development, 11, 458-462 (2007)
Paolo Tosatti et al.
The Journal of organic chemistry, 76(13), 5495-5501 (2011-05-14)
The sequential use of Cu-catalyzed asymmetric allylic alkylation, olefin cross-metathesis, and Ir-catalyzed asymmetric allylic amination allows the concise, stereodivergent synthesis of complex chiral amines with complete regiocontrol and good diastereoselectivity, exemplified by the synthesis of a pair of diastereoisomeric unnatural
Functionalised resin-modified carbon paste sensor for the voltammetric determination of Pb (II) within a wide concentration range.
Mikysek T, et al.
Electrochemical Communications, 10(2), 242-245 (2008)
Michael J. Girgis, et al.
Organic Process Research & Development, 12, 1209-1217 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service