358894
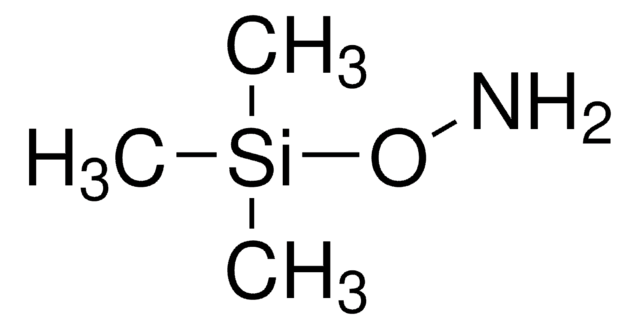
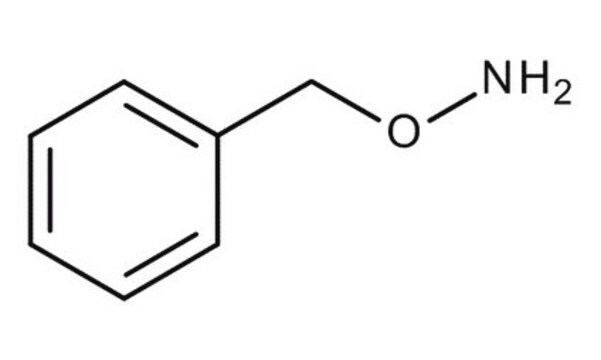
O-Tritylhydroxylamine
95%
Synonym(s):
O-(Triphenylmethyl)hydroxylamine, Trityloxyamine
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(C6H5)3CONH2
CAS Number:
Molecular Weight:
275.34
Beilstein:
1983917
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
mp
79-81 °C (lit.)
SMILES string
NOC(c1ccccc1)(c2ccccc2)c3ccccc3
InChI
1S/C19H17NO/c20-21-19(16-10-4-1-5-11-16,17-12-6-2-7-13-17)18-14-8-3-9-15-18/h1-15H,20H2
InChI key
NZFHJBSDSXDUAO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
O-Tritylhydroxylamine may be used in the preparation of 8-azido-O-trityloctahydroxamate and 9-azido-O-tritylnonahydroxamate. It may be used in the synthesis of Simian virus nuclear localization peptide (NLS)-histone deacetylase (HDAC) inhibitor conjugates.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Joshua C Canzoneri et al.
Bioorganic & medicinal chemistry letters, 19(23), 6588-6590 (2009-10-27)
We describe herein the synthesis and characterization of a new class of histone deacetylase (HDAC) inhibitors derived from conjugation of a suberoylanilide hydroxamic acid-like aliphatic-hydroxamate pharmacophore to a nuclear localization signal peptide. We found that these conjugates inhibited the histone
Vishal Patil et al.
Bioorganic & medicinal chemistry, 18(1), 415-425 (2009-11-17)
Histone deacetylase inhibitors (HDACi) are endowed with plethora of biological functions including anti-proliferative, anti-inflammatory, anti-parasitic, and cognition-enhancing activities. Parsing the structure-activity relationship (SAR) for each disease condition is vital for long-term therapeutic applications of HDACi. We report in the present
Francisco J Prado-Prado et al.
Bioorganic & medicinal chemistry, 18(6), 2225-2231 (2010-02-27)
There are many of pathogen parasite species with different susceptibility profile to antiparasitic drugs. Unfortunately, almost QSAR models predict the biological activity of drugs against only one parasite species. Consequently, predicting the probability with which a drug is active against
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service