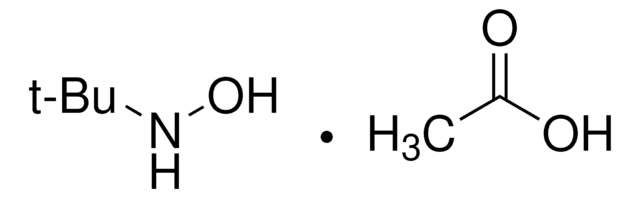20023
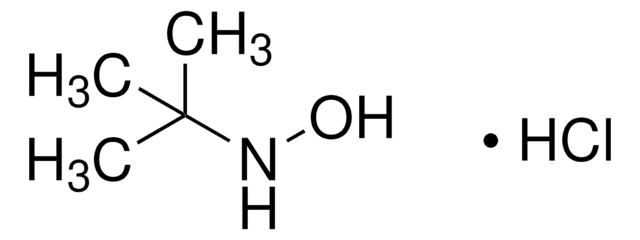
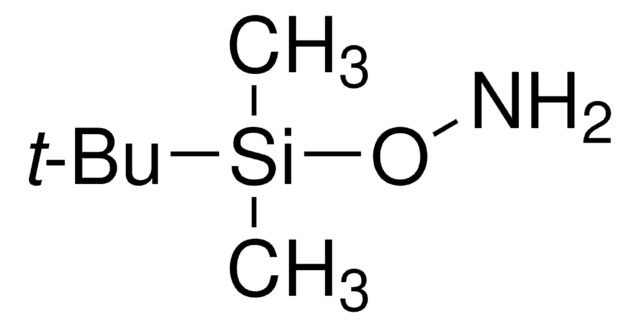
O-tert-Butylhydroxylamine hydrochloride
≥99.0% (AT)
Synonym(s):
2-Aminooxy-2-methylpropane hydrochloride, O-(1,1-Dimethylethyl)hydroxylamine hydrochloride, tert-Butoxyamine hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)3CONH2 · HCl
CAS Number:
Molecular Weight:
125.60
Beilstein:
3668106
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99.0% (AT)
form
solid
mp
~155 °C (dec.)
solubility
H2O: soluble 0.5 g/10 mL
SMILES string
Cl.CC(C)(C)ON
InChI
1S/C4H11NO.ClH/c1-4(2,3)6-5;/h5H2,1-3H3;1H
InChI key
ZBDXGNXNXXPKJI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
O-tert-Butylhydroxylamine hydrochloride was used in the synthesis of N-methyl-O-tert-butylhydroxylamine hydrochloride. It was also used in the preparation of N-tert-butoxyamino acids as substrates for the unambiguous synthesis of N-hydroxy peptides.
Reactant involved in synthesis of biologically active molecules including:
Reactant involved in:
- CGS 25966 derivatives for use as MMP inhibitors
- Imidazolidinedione derivatives for use as antimalarial treatments
- Pyrimidine ribonucleotide analogs as P2Y6 receptor agonists
- Rab proteins for isoprenylation and geranylgeranylation inhibition
Reactant involved in:
- Synthesis of N-(arylethyl)-O-tert-butylhydroxamates for use as Weinreb amide equivalents
- Double allylic alkylation of indole-2-hydroxamates
- SN2 substitution reactions at amide nitrogens
- Photocycloaddition to C=N bonds for synthesis of 1,3-diazepines
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Sol. 2
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Klaus Kopka et al.
Nuclear medicine and biology, 31(2), 257-267 (2004-03-12)
Non-invasive measurement of matrix metalloproteinase (MMP) activity in vivo is a clinical challenge in many disease processes such as inflammation, tumor metastasis and atherosclerosis. Therefore, radioiodinated analogues of the non-peptidyl broad-spectrum MMP inhibitor (MMPI) CGS 27023A 1a were synthesized for
T. Kolasa et al.
Tetrahedron, 30, 3591-3591 (1974)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service