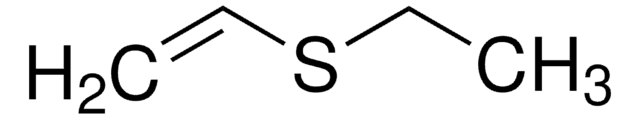All Photos(1)
About This Item
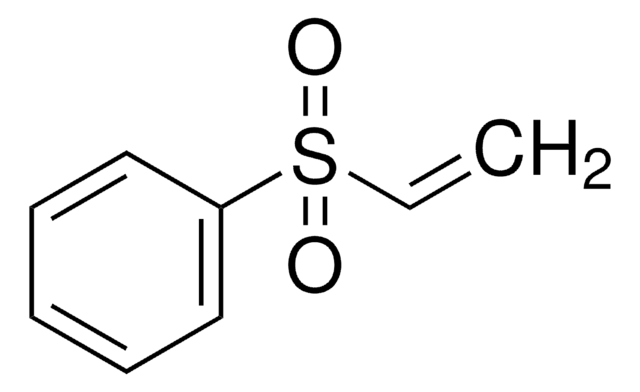
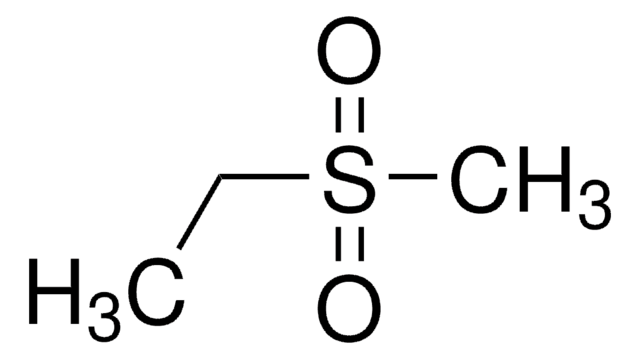
Linear Formula:
C2H5S(O)2CH=CH2
CAS Number:
Molecular Weight:
120.17
Beilstein:
1745130
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.463 (lit.)
bp
118-119 °C/22 mmHg (lit.)
density
1.151 g/mL at 25 °C (lit.)
SMILES string
CCS(=O)(=O)C=C
InChI
1S/C4H8O2S/c1-3-7(5,6)4-2/h3H,1,4H2,2H3
InChI key
BJEWLOAZFAGNPE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Ethly vinyl sulfone alkylates ε-amino groups of lysine side chains and imidazole groups of histidine residues in proteins. Chemical modification of bovine serum albumin by ethyl vinyl sulfone has been studied by X-ray photoelectron spectroscopy.
related product
Product No.
Description
Pricing
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
228.2 °F - closed cup
Flash Point(C)
109 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M Friedman et al.
International journal of peptide and protein research, 7(6), 481-486 (1975-01-01)
Ethly vinyl sulfone (EVS) alkylates xi-amino groups of lysine side chains and imidazole groups of histidine residues in proteins. Amino acid analysis of hydrolyzates of EVS-treated polylysine shows that lysine forms two derivatives, presumably xi-N-(ethylsulfonylethyl)lysine and xi, xi, N,N-bis(ethylsulfonylethyl)lysine that
X-ray photoelectron spectroscopy of BSA and ethyl vinyl sulfone modified BSA.
M M Millard et al.
Biochemical and biophysical research communications, 70(2), 445-451 (1976-05-17)
M S Masri et al.
Journal of protein chemistry, 7(1), 49-54 (1988-02-01)
Disulfide bonds of bovine serum albumin and wool were reduced by n-tributylphosphine to sulfhydryl groups that were then modified by methyl or ethyl vinyl sulfone in a nucleophilic addition reaction to S-(beta-ethylsulfonylmethyl)-L-cysteine and S(beta-ethylsulfonylethyl)-L-cysteine, respectively. The reductive alkylation was carried
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service