709980
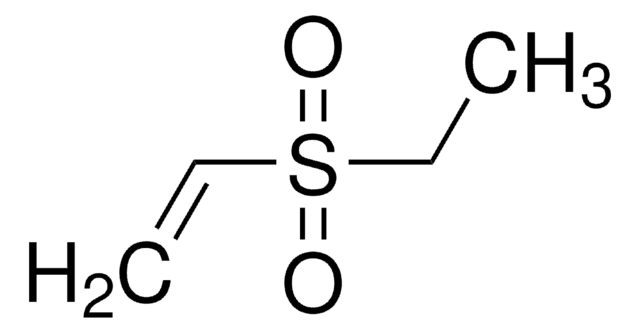
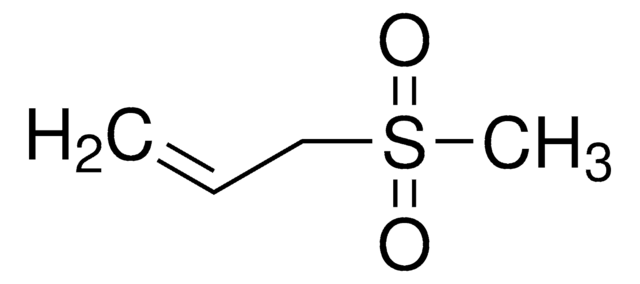
Ethyl methyl sulfone
for energy applications, 97%
About This Item
Recommended Products
Quality Level
Assay
97%
form
solid
bp
239 °C (lit.)
mp
32-37 °C
density
1.1 g/cm3
application(s)
battery manufacturing
SMILES string
CCS(C)(=O)=O
InChI
1S/C3H8O2S/c1-3-6(2,4)5/h3H2,1-2H3
InChI key
YBJCDTIWNDBNTM-UHFFFAOYSA-N
Related Categories
related product
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
>230.0 °F - closed cup
Flash Point(C)
> 110 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Dr. Schmuch, Dr. Siozios, Professor Dr. Winter, and Dr. Placke review the challenges and opportunities of nickelrich layered oxide cathode materials. They discuss production processes for the layered oxide cathode materials as well as their chemistry and morphology.
Research and development of solid-state lithium fast-ion conductors is crucial because they can be potentially used as solid electrolytes in all-solid-state batteries, which may solve the safety and energy-density related issues of conventional lithium-ion batteries that use liquid (farmable organic) electrolytes.
Lithium-ion batteries (LIBs) have been widely adopted as the most promising portable energy source in electronic devices because of their high working voltage, high energy density, and good cyclic performance.
The critical technical challenges associated with the commercialization of electric vehicle batteries include cost, performance, abuse tolerance, and lifespan.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service