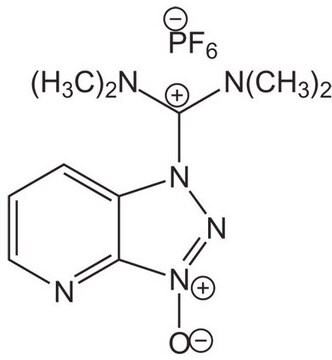
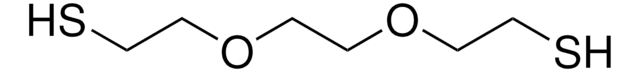
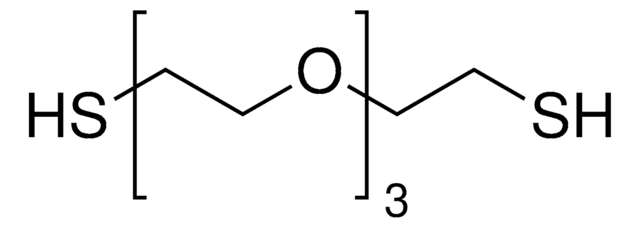
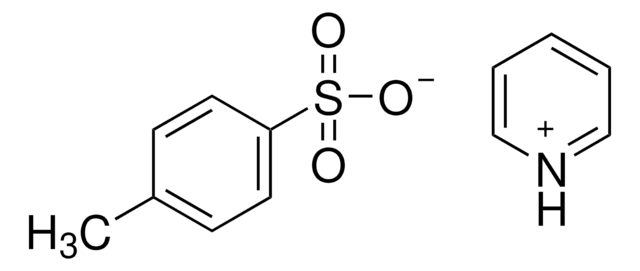
105449
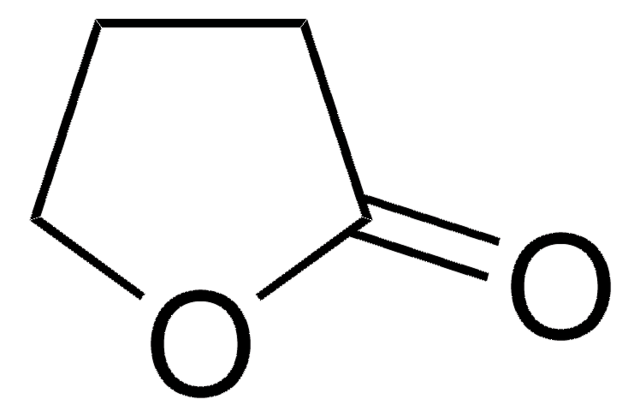
γ-Thiobutyrolactone
98%
Synonym(s):
4-Butyrothiolactone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H6OS
CAS Number:
Molecular Weight:
102.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.523 (lit.)
bp
39-40 °C/1 mmHg (lit.)
solubility
THF: soluble
density
1.18 g/mL at 25 °C (lit.)
SMILES string
O=C1CCCS1
InChI
1S/C4H6OS/c5-4-2-1-3-6-4/h1-3H2
InChI key
KMSNYNIWEORQDJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
γ-Thiobutyrolactone undergoes copolymerization with glycidyl phenyl ether to form poly(ester-alt-sulfide).
Application
γ-Thiobutyrolactone was used to terminate the ring opening polymerization of ω-pentadecalactone to synthesize difunctional polyesters. γ-Thiobutyrolactone was used to study the mechanism of metabolism of sulphur containing heterocyclic compounds by lignin-degrading basidiomycete Coriolus versicolor.
Signal Word
Warning
Hazard Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
186.8 °F - closed cup
Flash Point(C)
86 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tiny droplets make a big splash.
Michael Eisenstein
Nature methods, 3(2), 71-71 (2006-02-14)
Jonathan Garel et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 12(15), 4144-4152 (2006-02-03)
Homocysteine thiolactone (tHcy) is deemed a risk factor for cardiovascular diseases and strokes, presumably because it acylates the side chain of protein lysine residues ("N-homocysteinylation"), thereby causing protein damage and autoimmune responses. We analysed the kinetics of hydrolysis and aminolysis
Nishikubo et al.
Macromolecules, 31(15), 4746-4752 (1998-07-29)
Poly(ester-alt-sulfide) (polymer 1) was synthesized by the alternating copolymerization of glycidyl phenyl ether (GPE) with gamma-thiobutyrolactone (TBL) catalyzed by either quaternary onium salts or crown ether complexes. The copolymerization proceeded to produce polymer 1 with good yields in neat or
K D Holland et al.
Brain research, 615(1), 170-174 (1993-06-25)
Effects of alkyl-substituted gamma-butyrolactones and gamma-thiobutyrolactones on [35S]t-butylbicyclophosphorothionate (35S-TBPS) dissociation from the picrotoxinin receptor were studied. Unlike picrotoxinin, these lactones accelerated the dissociation rate of 35S-TBPS. Thus, previous reports that these lactones change the Kd but not the Bmax of
One-pot difunctionalization of poly (ω-pentadecalactone) with thiol-thiol or thiol-acrylate groups, catalyzed by Candida antarctica lipase B.
Takwa M, et al.
Macromolecular Rapid Communications, 27(22), 1932-1936 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service