143049
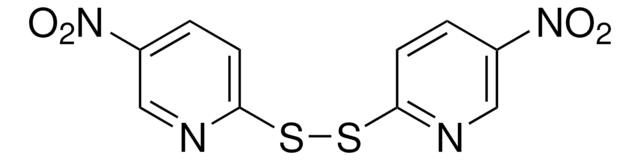
Aldrithiol™-2
98%
Synonym(s):
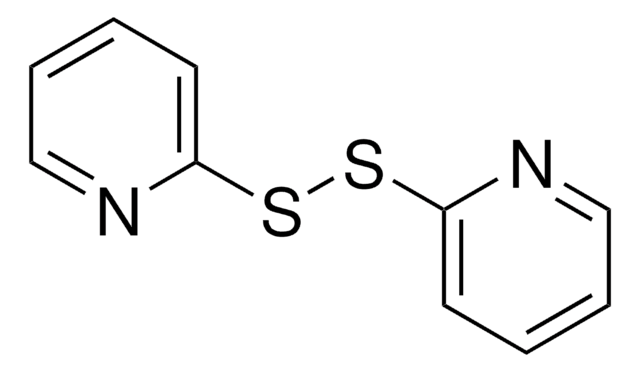
2,2′-Dithiodipyridine, 2,2′-Dipyridyl disulfide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H8N2S2
CAS Number:
Molecular Weight:
220.31
Beilstein:
154629
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
56-58 °C (lit.)
solubility
methanol: soluble 50 mg/mL, clear to very slightly hazy, colorless to faintly brownish-yellow
storage temp.
2-8°C
SMILES string
S(Sc1ccccn1)c2ccccn2
InChI
1S/C10H8N2S2/c1-3-7-11-9(5-1)13-14-10-6-2-4-8-12-10/h1-8H
InChI key
HAXFWIACAGNFHA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Aldrithiol™-2 is a nucleocapsid zinc finger targeting compound and can abrogate the infectivity of human immunodeficiency virus type-1 virions. It is a thiol reagent.
Application
Aldrithiol™-2 was used in the synthesis of adenosine-5′-phosphoimidazolide. It was employed with PPh3 as a condensing agent in the amidation of carboxylic acid enantiomers for HPLC separation.
Thiol reagent. Employed with PPh3 as a condensing agent in the amidation of carboxylic acid enantiomers for HPLC separation.
Legal Information
Aldrithiol is a trademark of Sigma-Aldrich Co. LLC
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tetrahedron Letters, 35, 1267-1267 (1994)
Analytical Sciences, 10, 17-17 (1994)
J L Rossio et al.
Journal of virology, 72(10), 7992-8001 (1998-09-12)
Whole inactivated viral particles have been successfully used as vaccines for some viruses, but procedures historically used for inactivation can denature virion proteins. Results have been inconsistent, with enhancement of disease rather than protection seen in some notable instances following
Journal of Chromatography A, 662, 87-87 (1994)
T L Dutka et al.
The Journal of physiology, 589(Pt 9), 2181-2196 (2010-12-01)
S-Nitrosoglutathione (GSNO) is generated in muscle and may S-glutathionylate and/or S-nitrosylate various proteins involved in excitation–contraction (EC) coupling, such as Na+-K+-ATPases, voltage-sensors (VSs) and Ca2+ release channels (ryanodine receptors,RyRs), possibly changing their properties. Using mechanically skinned fibres from rat extensor
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service