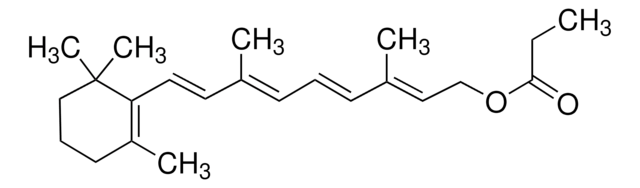
R4632
Retinyl acetate
synthetic, crystalline solid or supercooled liquid
Synonym(s):
Retinol acetate, Vitamin A acetate
About This Item
Recommended Products
biological source
synthetic
Quality Level
Assay
≥90% (HPLC)
form
crystalline solid or supercooled liquid
potency
2,600,000-2,940,000 I.U. per g
color
light yellow to dark yellow
mp
57-58 °C
~58 °C (lit.)
storage temp.
−20°C
SMILES string
CC1=C(/C=C/C(C)=C/C=C/C(C)=C/COC(C)=O)C(C)(C)CCC1
InChI
1S/C22H32O2/c1-17(9-7-10-18(2)14-16-24-20(4)23)12-13-21-19(3)11-8-15-22(21,5)6/h7,9-10,12-14H,8,11,15-16H2,1-6H3/b10-7+,13-12+,17-9+,18-14+
InChI key
QGNJRVVDBSJHIZ-QHLGVNSISA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- to study its effects on cochlear development in mice
- as a component of an internal standard to quantify antioxidants in yolk
- as an internal standard to determine serum retinol concentration
Biochem/physiol Actions
Retinoids also exist as retinyl esters such as retinyl propionate, retinyl acetate and retinyl palmitate. Retinyl esters provide pools of vitamin A that are converted into retinol and other retinoids as needed. Retinyl acetate is used in a wide range of biological applications.
Physical form
Analysis Note
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 4 - Repr. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
All-trans retinoic acid (RA, ATRA) is a pleiotropic activation factor that regulates genes associated with normal vertebrate cellular processes such as cell differentiation, cell proliferation, apoptosis, and embryonic development.
Protocols
Following GB method for Vitamin A and Vitamin E as it is written with a 250 x 4.6 mm Ascentis® Express C30 column gives baseline resolution between the critical pair (gamma-, and beta-tocopherol; peaks 3 and 4).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service