520160
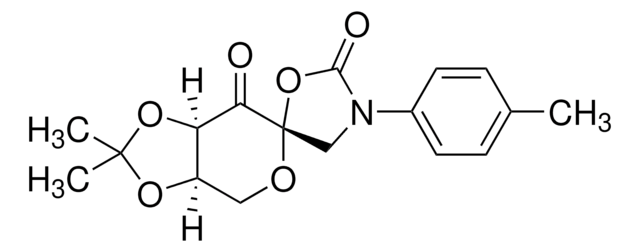
Shi Epoxidation Diketal Catalyst
98%
Synonym(s):
1,2:4,5-Di-O-isopropylidene-β-D-erythro-2,3-hexodiulo-2,6-pyranose
About This Item
Recommended Products
Quality Level
Assay
98%
optical activity
[α]20/D −120.9°, c = 1 in chloroform
greener alternative product characteristics
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
102-104 °C (lit.)
functional group
ether
ketal
ketone
greener alternative category
, Aligned
SMILES string
CC1(C)O[C@@H]2CO[C@]3(COC(C)(C)O3)C(=O)[C@@H]2O1
InChI
1S/C12H18O6/c1-10(2)15-6-12(18-10)9(13)8-7(5-14-12)16-11(3,4)17-8/h7-8H,5-6H2,1-4H3/t7-,8-,12+/m1/s1
InChI key
IVWWFWFVSWOTLP-RWYTXXIDSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
An Efficient Catalytic Asymmetric Epoxidation Method
Use of an Iridium-Catalyzed Redox-Neutral Alcohol-Amine Coupling on Kilogram Scale for the Synthesis of a GlyT1 Inhibitor
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
We presents an article regarding asymmetric epoxidation using Shi catalyst.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service