320617
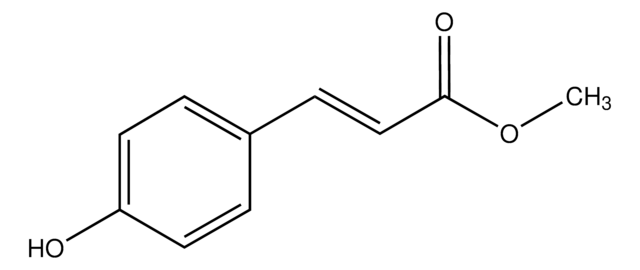
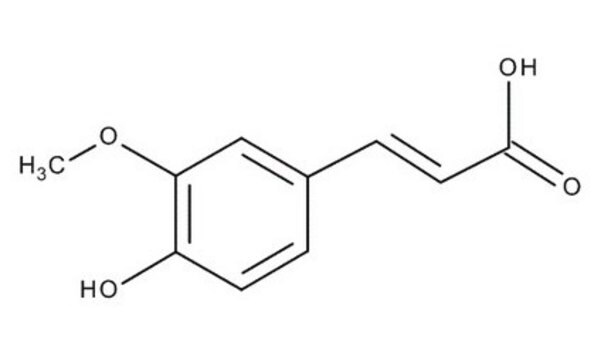
Ethyl 4-hydroxy-3-methoxycinnamate
98%
Synonym(s):
Ethyl ferulate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOC6H3(OCH3)CH=CHCO2C2H5
CAS Number:
Molecular Weight:
222.24
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
bp
164-166 °C/0.5 mmHg (lit.)
mp
63-65 °C (lit.)
functional group
ester
SMILES string
CCOC(=O)\C=C\c1ccc(O)c(OC)c1
InChI
1S/C12H14O4/c1-3-16-12(14)7-5-9-4-6-10(13)11(8-9)15-2/h4-8,13H,3H2,1-2H3/b7-5+
InChI key
ATJVZXXHKSYELS-FNORWQNLSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
J Donaghy et al.
Applied microbiology and biotechnology, 50(2), 257-260 (1998-10-09)
The production of feruloyl esterase activity by Bacillus spp. and lactobacilli can be detected in an agarplate assay. The assay involves the substitution of the main carbon source in specific agar with ethyl ferulate. A number of Bacillus spp., predominantly
T Nakayama et al.
Bioscience, biotechnology, and biochemistry, 60(2), 316-318 (1996-02-01)
Cytotoxicity and DNA single-strand breaks caused by H2O2 were assessed by a colony formation assay and a DNA precipitation assay, respectively, with Chinese hamster V79 cells. In both assays, caffeic acid ethyl ester showed protective effects. The structure-activity relationship showed
K J Rashamuse et al.
Journal of applied microbiology, 103(5), 1610-1620 (2007-10-24)
Isolation and identification of bacterial isolates with specific ferulic acid (FA) esterase activity and cloning of a gene encoding activity. A micro-organism with ethyl ferulate hydrolysing (EFH) activity was isolated by culture enrichment techniques. Detailed molecular identification based on species-specific
Liang Kong et al.
Analytical and bioanalytical chemistry, 386(2), 264-274 (2006-07-27)
A new screening and analysis method that combines in vitro metabolism with high-performance liquid chromatography-mass spectrometry (HPLC-MS) was developed for the screening and analysis of an antineoplastic compound, coniferyl ferulate, which is present in the rhizome of Rhizoma Chuanxiong. Infrared
Yun Feng et al.
Biochimica et biophysica acta, 1780(4), 659-672 (2008-01-31)
It is an important therapeutic strategy to protect mitochondria from oxidative stress, especially during ischemia-reperfusion. In the present study, an attempt has been made to evaluate the protective effects of caffeic acid phenethyl ester (CAPE) and its related phenolic compounds
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service