RT-qPCR, or quantitative reverse transcription PCR, combines the effects of reverse transcription and quantitative PCR or real-time PCR to amplify and detect specific targets. RT-qPCR has a variety of applications including quantifying gene expression levels, validating RNA interference (RNAi), and detecting pathogens such as viruses. Common approaches to generate a fluorescent signal used for measuring DNA quantity in this technique are to use either hydrolysis probes such as TaqMan® probes, or a double-stranded DNA binding dye such as SYBR® Green dye.
What is RT-qPCR?
Quantitative reverse transcription PCR (RT-qPCR) involves the detection and quantification of RNA. The process is performed by reverse transcription of total RNA or mRNA to complementary DNA (cDNA) by the enzyme reverse transcriptase, followed by amplification and detection of specific targets of this cDNA using a technique called quantitative PCR (qPCR) or real-time PCR. At each cycle during this PCR, the quantity of DNA is measured in real-time by using a variety of fluorescent chemistries. The most common approaches to generate a fluorescent signal are to use either hydrolysis probes such as TaqMan® probes, or a double-stranded DNA binding dye such as SYBR® Green dye.
The selection of fluorescent chemistry is dependent upon factors such as the application, cost, and whether the assay is a singleplex or multiplex assay. DNA-binding dyes are preferred for singleplex, low-throughput assays since they are easier to design, have lesser set-up time, and are more cost-efficient. Fluorescent probes are more commonly employed in high-throughput, multiplex assays that require higher specificity.
For more information on qPCR, view our Quantitative PCR Technical Guide
Applications of RT-qPCR
RT-qPCR has a variety of applications. The RT-qPCR technique is used to:
- quantify gene expression levels
- validate RNAi to study loss of function of selective genes
- detect pathogens such as viruses for the diagnosis of infectious diseases
- detect genetically modified organisms (GMOs)
One-Step RT-qPCR vs Two-Step RT-qPCR
RT-qPCR is performed in either a one-step or a two-step assay. It is important to consider various factors like cost, application, and assay type when choosing the right kind of RT-qPCR method.

Figure 1. Diagram comparing one-step RT-qPCR workflow vs two-step RT-qPCR workflow.
One-Step RT-qPCR
In one-step RT-qPCR, reverse transcription or cDNA synthesis and qPCR are carried out in a single tube by employing sequence-specific primers for the amplification of a specific target. Genetically modified reverse transcriptases are commonly employed in one-step assays as they tolerate higher temperatures necessary for annealing of sequence-specific primers. A one-step assay is preferred when repeated quantification of the same gene is carried out, especially in high-throughput and diagnostic applications. Another consideration when choosing one-step assays is the quality and scarcity of RNA. Since the cDNA synthesized during the reaction cannot be saved separately as an aliquot, more samples of the original RNA may be required to repeat the assay.
Two-Step RT-qPCR
In two-step RT-qPCR, reverse transcription and qPCR reactions are carried out in separate tubes having separate buffers and reagents. Random hexamers, oligo-dT primers, and/or gene-specific primers may be used in two-step assays. Having separate buffers and reagents allows more flexibility in choosing the reverse transcriptase enzymes and PCR reagents, with more options to optimize and improve the amplification efficiency of difficult templates. The more stable cDNA that is synthesized in the first step can be further concentrated and/or purified to be either stored away for future use or it may be used for the quantification of multiple genes from the same sample.
| Assay Type | Primers Used | Key Features |
|---|---|---|
| One-Step RT-qPCR | Sequence-specific primers |
|
| Two-Step RT-qPCR | Oligo (dT) primers, random hexamers, and/or sequence-specific primers |
|
Explore more on PCR assay optimization in our Assay Optimization and Validation page
One-Step RT-qPCR Products for High-Throughput Applications
Our one-step RT-qPCR products combine the effect of reverse transcriptase with hot-start Taq-directed antibody in convenient ReadyMixTM PCR reaction mixes for probe-based or SYBR® Green based applications. Regardless of difficult secondary structures or detection chemistry, our ReadyMixTM PCR reaction mixes are specially formulated to help achieve superior results in fewer steps.
Our products will easily fit into your PCR workflow and with a variety of dependable products for one-step RT-qPCR experiments – you won’t make a wrong choice. Discover KiCqStart® products and find a ReadyMix™ master mix optimized for your instrument or explore our JumpStart™ options for kits optimizable to your experimental needs. For high-throughput, fast-cycling, one-step RNA quantification using sequence-specific fluorogenic probes choose the KAPA PROBE FAST universal kit. Confidently choose any of our RT-qPCR products without compromising on quality.
One-Step Probe RT-qPCR ReadyMix™ KiCqStart® – Compatible with all major qPCR instruments
Our KiCqStart® One-Step Probe RT-qPCR ReadyMix™ reagents feature a stringent hot-start mechanism for greater specificity and are formulated to be highly tolerant of PCR inhibitors in samples. These highly sensitive ready-to-use master mixes are designed for convenience and contain all required basic components for qPCR or RT-qPCR. Simply add your primers, probe, and water to complete the assay cocktail. This product is ideal for fast or conventional qPC
| Cat. No. | Product Name | Features |
|---|---|---|
| KCQS07 | KiCqStart® One-Step Probe RT-qPCR ReadyMix™ | For Bio-Rad®, Cepheid®, Eppendorf®, Illumina®, Corbett®, and Roche® Instruments |
| KCQS08 | KiCqStart® One-Step Probe RT-qPCR ReadyMix™, Low ROX™ | For Applied Biosystems® (ABI) and Stratagene® instruments |
| KCQS09 | KiCqStart® One-Step Probe RT-qPCR ReadyMix™, ROX | For Applied Biosystems® (ABI) instruments |
Learn more about instrument compatibility with these products in our Full Instrument Compatibility Chart or discover our Custom qPCR Probes.
JumpStart™ Taq ReadyMix™ – Optimizable for all major qPCR instruments
Our JumpStart™ Taq ReadyMix™ products for RT-qPCR combines the advantages of the Moloney Murine Leukemia Virus Reverse Transcriptase (M-MLV RT) and our JumpStart™ antibody-inactivated Taq polymerase. Robust M-MLV RT has the ability to transcribe through difficult secondary structures, even at elevated temperatures while our JumpStart™ Taq polymerase provides greater specificity and sensitivity than standard Taq polymerase. These products are compatible with both tube and plate-based instruments as well as capillary-based instruments.
Save 20% on RT-qPCR ReadyMix™ (QR0200) by using code SGX at checkout. Valid until January 31, 2021.
KAPA PROBE FAST – Compatible with all fluorogenic probe-based technologies
The KAPA PROBE FAST One-Step qRT-PCR Master Mix Universal Kit is a sensitive and convenient solution for real-time PCR using RNA as template. The kit is designed for high throughput, fast-cycling, one-step RNA quantification using sequence-specific fluorogenic probes. It is compatible with all fluorogenic probe-based technologies, including hybridization probes (e.g., fluorescence resonance energy transfer (FRET) probes), hydrolysis probes (e.g., TaqMan® probes), and displacement probes (e.g., molecular beacons).
This kit is a ready-to-use cocktail containing all components except primers, probe(s), and template for fast-cycling, probe-based real-time PCR. The kit contains KAPA PROBE FAST qPCR Master Mix, ROX High and ROX Low Reference Dyes, KAPA RT Mix, and dUTP.
| Cat. No. | Product Name | Features |
|---|---|---|
| KK4752 | KAPA PROBE FAST One-Step qRT-PCR Master Mix (2X) Universal Kit | High-throughput, fast-cycling, one-step RNA quantification using sequence-specific fluorogenic probes |
ABScript II One Step RT-qPCR Probe Kit – Compatible with all major qPCR instruments
The ABScript II Reverse Transcriptase in the kit provides reliable reverse transcription of RNA. After reverse transcription, the hot-start version of Taq polymerase is activated at 95 °C and the ABScript II Reverse Transcriptase is inactivated simultaneously. In the sequential PCR reaction, the 5′-3′ exonuclease activity of Taq polymerase cleaves the hybridized probe, separating the reporter from the quencher and releasing a fluorescent signal.
The ABScript II One Step RT-qPCR Probe Kit is a ready-to-use kit for reverse transcription and subsequent probe-based qPCR in a single tube. This kit’s flexible format includes two separate 50X concentrations of the ROX (passive reference) dye, to be added as required by specific qPCR instruments.
| Cat. No. | Product Name | Features |
|---|---|---|
| RK20407 | ABScript II One Step RT-qPCR Probe Kit | Analyzes low input RNA samples, low contamination risk |
For Research Use Only. Not For Use In Diagnostic Procedures.
Two-Step RT-qPCR Products for Higher Flexibility
We offer all the reagents needed to perform reverse transcription followed by PCR analysis of the synthesized cDNA in separate tubes for higher flexibility in your experiments. Confidently incorporate our two-step RT-qPCR products in your PCR workflow without compromising on flexibility. With various options available for our reliable PCR products, you won’t make a wrong choice.
cDNA Synthesis Kits and Mixes
To meet your reverse transcription needs, choose from various types of enzymes and several packaging options. All these products include the reagents necessary for first strand synthesis and are available as kits with separate reagents for optimization or as complete mixes for quick and convenient First-Strand synthesis.
| Cat. No. | Product Name | Features |
|---|---|---|
| STR1 | Enhanced Avian First-Strand Synthesis Kit |
|
| 11483188001 | First Strand cDNA Synthesis Kit for RT-PCR |
|
| RDRT | ReadyScript™ cDNA Synthesis Mix |
|
| 11117831001 | cDNA Synthesis Kit |
|
| THIFICDNARO | Transcriptor High Fidelity cDNA Synthesis Kit |
|
| 5893151001 | Transcriptor Universal cDNA Master |
|
RT Enzymes — Reverse Transcriptases
Choose from our collection of RT enzymes and find one that suits the complexity of your RNA transcript. Our products include standard Avian Myeloblastosis Virus (AMV) and Moloney Murine Leukemia Virus (M-MLV) Reverse Transcriptases as well as enhanced versions of these for higher sensitivity.
| Cat. No. | Product Name | Features |
|---|---|---|
| A4464 | Enhanced Avian Reverse Transcriptase |
|
| ERT-RO | Expand™ Reverse Transcriptase |
|
| M1302 | M-MLV Reverse Transcriptase |
|
| 10109118001 | Reverse Transcriptase AMV |
|
| 11062603001 | Reverse Transcriptase M-MuLV |
|
| TRANSRTRO | Transcriptor Reverse Transcriptase |
|
Explore more about RT-qPCR troubleshooting in RT-PCR / RT-qPCR Troubleshooting page.
qPCR Kits
These ReadyMixTM products contain all the necessary components for qPCR. Simply add the fluorescent detection chemistry, primers, and template.
Probe Based qPCR
Probe based qPCR relies on the sequence-specific detection of a desired PCR product. Unlike SYBR® Green based qPCR methods that detect all double-stranded DNA, probe-based qPCR utilizes a fluorescent-labeled target-specific probe resulting in increased specificity and sensitivity. Additionally, a variety of fluorescent dyes are available so that multiple primers can be used to simultaneously amplify many sequences.
| Cat. No. | Product Name | Features |
|---|---|---|
| D6442 | JumpStart™ Taq ReadyMix™ for High Throughput Quantitative PCR |
|
| D7440 | JumpStart™ Taq ReadyMix™ for Quantitative PCR |
|
| L6669 | LuminoCt® qPCR ReadyMix™ |
|
| FSTMPMMRO | FastStart TaqMan® Probe Master |
|
| FSUPMMRO | FastStart Universal Probe Master (Rox) |
|
| PFUKB | KAPA PROBE FAST qPCR Master Mix, Universal |
|
SYBR® Green Based qPCR
SYBR® Green I, a commonly used fluorescent DNA binding dye, binds all double-stranded DNA. Detection is monitored by measuring the increase in fluorescence throughout the cycle. SYBR® Green I has an excitation and emission maxima of 494 nm and 521 nm, respectively. Specificity of our SYBR® Green based qPCR detection is greatly enhanced by the incorporation of a hot start mediated Taq polymerase, like JumpStart™ Taq.
| Cat. No. | Product Name | Features |
|---|---|---|
| KCQS02 | KiCqStart® SYBR® Green qPCR ReadyMix™ with ROX™ |
|
| KCQS01 | KiCqStart® SYBR® Green qPCR ReadyMix™ Low ROX™ |
|
| KCQS00 | KiCqStart® SYBR® Green qPCR ReadyMix™ |
|
| KCQS03 | KiCqStart® SYBR® Green qPCR ReadyMix™ iQ™ |
|
| S4438 | SYBR® Green JumpStart™ Taq ReadyMix™ |
|
| S5193 | SYBR® Green JumpStart™ Taq ReadyMix™ |
|
| S9194 | SYBR® Green JumpStart™ Taq ReadyMix™ for High Throughput qPCR |
|
| S1816 | SYBR® Green JumpStart™ Taq ReadyMix™ for Quantitative PCR |
|
| SFUKB | KAPA SYBR® FAST qPCR Master Mix, Universal |
|
| FSUSGMMRO | FastStart Universal SYBR® Green Master (Rox) |
|
| L6544 | LuminoCt® SYBR® Green qPCR ReadyMix™ |
|
Discover more on SYBR® Green based qPCR in this short video.
MicroRNA-Based RT-qPCR
Find the microRNA reverse transcription products you need with our MystiCq® product line.
MystiCq® MicroRNA RT-qPCR SystemR
MystiCq® microRNA reagents provide a complete SYBR® Green RT-qPCR workflow for quantifying expression in just three steps:
- Isolation
- cDNA synthesis
- Quantitation by amplification
Discover more about the MystiCq® workflow in our MystiCq® product page. These products feature:
- Over 1,400 pre-designed and wet lab tested primer pairs designed to target only mature microRNAs
- Conversion of all microRNAs, allowing nearly unlimited readouts from a single sample
- Selection of three different products for isolation of microRNA
qPCR Assay Designs and Reagents for SARS-CoV-2 Research
We are committed to providing researchers with reliable resources and tools to support the fight against COVID-19 (SARS-CoV-2). In response to the community’s need for SARS-CoV-2-specific reagents and protocols, our scientists have evaluated the RNA-based One-Step RT-qPCR kits for SARS-CoV-2 target detection. For Research Use Only. Not For Use In Diagnostic Procedures.
Experimental Design and Methods
This experiment was conducted to determine if our One-Step RT-qPCR kits can detect SARS-CoV-2. The assay was performed following the CDC protocol for SARS-CoV-2 detection using synthesized SARS-Cov-2 RNA as template, diluted into 105, 104, 103, 102, and 10 copies. The test sample contained the template, the reagents from each kit, target-specific DNA primers /TaqMan® probe sets for N1 and N2 (N, nucleocapsid gene). NTC (no template control) samples were also included in each assay.
| Primer Target | F Primer 5'-3' | R Primer, 5'-3' | Probe | Amplicon Size |
|---|---|---|---|---|
| N1 | GACCCCAAAATCAGCGAAAT | TCTGGTTACTGCCAGTTGAATCTG | 5'-FAM-ACC CCG CAT TAC GTT TGG TGG ACC-BHQ1-3' | 71 bp |
| N2 | TTACAAACATTGGCCGCAAA | GCGCGACATTCCGAAGAA | 5'-FAM-ACA ATT TGC CCC CAG CGC TTC AG-BHQ1-3' | 67 bp |
Table 2. The primer and probe sets designed for specific detection of SARS-CoV-2 (N1 and N2 assays). For Research Use Only. Not For Use In Diagnostic Procedures.
Test Results
A: N1 Assay

B: N2 Assay

Figure 2. SARS-CoV-2 detection assays utilizing the Quantitative RT-PCR ReadyMix™ (Cat. No. QR200) for primer targets N1 (graph A) and N2 (graph B).
The SARS-CoV-2 detection N1 (graph A) and N2 (graph B) assays in Figure 2 were performed with Quantitative RT-PCR ReadyMix™ (Cat. No. QR200) using synthesized SARS-CoV-2 RNA as template. Both assays showed sensitivities to detect as low as 102 copies of template. No amplification was observed with NTC samples
A: N1 Assay

B: N2 Assay

Figure 3. SARS-CoV-2 detection assays utilizing the KiCqStart® One-Step Probe RT-qPCR ReadyMix™ (Cat. No. KCQS07) for primer targets N1 (graph A) and N2 (graph B).
The SARS-CoV-2 detection N1 (graph A) and N2 (graph B) assays in Figure 3 were performed with KiCqStart® One-Step Probe RT-qPCR ReadyMix™ (Cat. No. KCQS07) using synthesized SARS-CoV-2 RNA as template. Both assays showed sensitivities to detect as low as 10 copies of template. No amplification was observed with NTC samples.
A: N1 Assay

B: N2 Assay

Figure 4. SARS-CoV-2 detection assays utilizing the KAPA PROBE FAST One-Step qRT-PCR Master Mix (2X) Universal Kit (Cat. No. KK4752) for primer targets N1 (graph A) and N2 (graph B).
The SARS-CoV-2 detection N1 (graph A) and N2 (graph B) assays in Figure 4 were performed with the KAPA PROBE FAST One-Step qRT-PCR Master Mix (2X) Universal Kit (Cat. No. KK4752) using synthesized SARS-CoV-2 RNA as template. Both assays showed sensitivities to detect as low as 102 copies of template. No amplification was observed with NTC samples.
A: N1 Assay
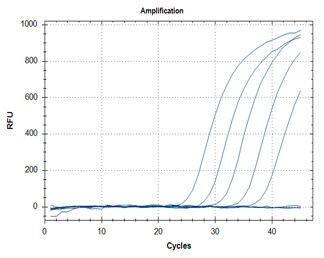
B: N2 Assay

Figure 5. SARS-CoV-2 detection assays utilizing the ABScript II One Step RT-qPCR Probe Kit (Cat. No. RK20407) for primer targets N1 (graph A) and N2 (graph B).
The SARS-CoV-2 detection N1 (graph A) and N2 (graph B) assays in Figure 5 were performed with the ABScript II One Step RT-qPCR Probe Kit (Cat. No. RK20407) using synthesized SARS-CoV-2 RNA as template. Both assays showed sensitivities to detect as low as 10 copies of template. No amplification was observed with NTC samples.
Conclusion
The above results indicate that our One-Step RT-qPCR reagents and kits are ideal for SARS-CoV-2 target detection in SARS-CoV-2 research. Based on this information, the assays using the One-Step RT-qPCR reagents can detect as low as 100 copies, or in some cases, as low as 10 copies of the RNA template.
For Research Use Only. Not For Use In Diagnostic Procedures.
The unpublished data presented in this technical article is from internal research.
For more information on RT-qPCR products for SARS-CoV-2 Detection, visit Sigma-Aldrich.com/covid19
More on qPCR assay design can be found in our Coronavirus qPCR Design Case Study & Related Products to Support SARS-CoV-2 Research article.
TRI Reagent® for Total RNA Isolation
TRI Reagent® is ideal for quick, economical, and efficient isolation of total RNA or the simultaneous isolation of RNA, DNA, and proteins from samples of human, animal, plant, yeast, bacterial, and viral origin.
This product, a mixture of guanidine thiocyanate and phenol in a monophase solution, effectively dissolves DNA, RNA, and protein on homogenization or lysis of tissue sample. After adding chloroform or 1-bromo-3-chloropropane and centrifuging, the mixture separates into 3 phases: an aqueous phase containing the RNA, the interphase containing DNA, and an organic phase containing proteins. Each component can then be isolated after separating the phases. One ml of TRI Reagent® is sufficient to isolate RNA, DNA, and protein from 50-100 mg of tissue, 5-10 ´ 106 cells, or 10 cm2 of culture dish surface for cells grown in monolayer.
This is one of the most effective methods for isolating total RNA and can be completed in only 1 hour starting with fresh tissue or cells. The procedure is very effective for isolating RNA molecules of all types from 0.1-15 kb in length. The resulting RNA is intact with little or no contaminating DNA and protein. It can be used for Northern blots, mRNA isolation, in vitro translation, RNase protection assay, cloning, and PCR.
TRI Reagent® has been extensively used in the inactivation of viruses and extraction of viral RNA for the detection of SARS-CoV-2 by RT-qPCR assays in several research studies.1, 2, 3 It has been shown to be ideal for the isolation and storage of mi-RNA at -800C for extended periods.4 It has been used in the extraction of feline and canine coronavirus RNA that is further used in RT-PCR assay.5, 6 It has also been used to isolate cytoplasmic RNA from SARS-CoV infected Vero cells. 7
| Cat. No. | Product Name | Features |
|---|---|---|
| T9424 | TRI Reagent® | For processing tissues, cells cultured in monolayer, or cell pellets |
For Research Use Only. Not For Use In Diagnostic Procedures.
References
*This article is a preprint and has not been peer-reviewed. It reports new medical research that has yet to be evaluated and so should not be used to guide clinical practice.
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?