H5257
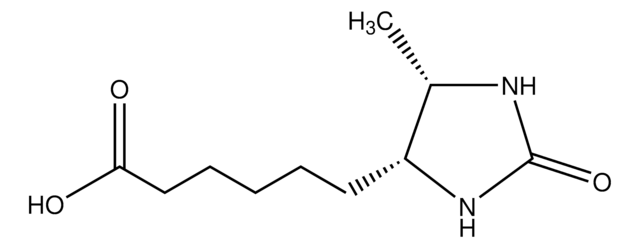
Hispidin
solid, ≥98% (HPLC)
Sinónimos:
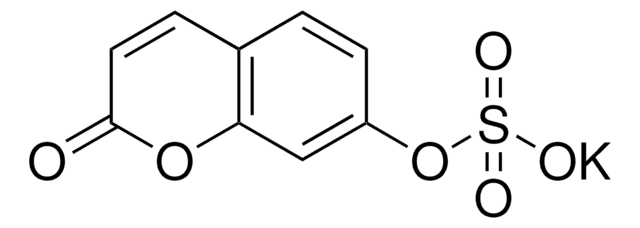
6-(3,4-dihydroxystyrl)-4-hydroxy-2-pyrone
About This Item
Productos recomendados
biological source
synthetic (organic)
assay
≥98% (HPLC)
form
solid
storage condition
protect from light
color
yellow to brown
mp
237.5-238.5 °C
solubility
DMSO: >10 mg/mL
storage temp.
−20°C
SMILES string
OC1=CC(/C=C/C(O2)=CC(O)=CC2=O)=CC=C1O
InChI
1S/C13H10O5/c14-9-6-10(18-13(17)7-9)3-1-8-2-4-11(15)12(16)5-8/h1-7,14-16H/b3-1+
InChI key
SGJNQVTUYXCBKH-HNQUOIGGSA-N
Gene Information
human ... PRKACB(5567) , PRKAR2B(5577) , PRKCB(5579)
General description
Biochem/physiol Actions
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
No data available
flash_point_c
No data available
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico