E5389
Erythromycin
powder, suitable for cell culture, BioReagent
Sinónimos:
E-Mycin, Erythrocin
About This Item
Productos recomendados
product name
Erythromycin, BioReagent, suitable for cell culture
product line
BioReagent
Quality Level
form
powder
potency
≥850 μg per mg
technique(s)
cell culture | mammalian: suitable
impurities
≤0.1 EU/mg endotoxin
color
white
mp
133 °C
solubility
2 M HCl: 50 mg/mL (Stock solutions should be stored at 2-8 °C. Stable at 37 °C for 3 days.)
ethanol: soluble (Stock solutions should be stored at 2-8 °C. Stable at 37 °C for 3 days.)
antibiotic activity spectrum
Gram-negative bacteria
Gram-positive bacteria
mode of action
protein synthesis | interferes
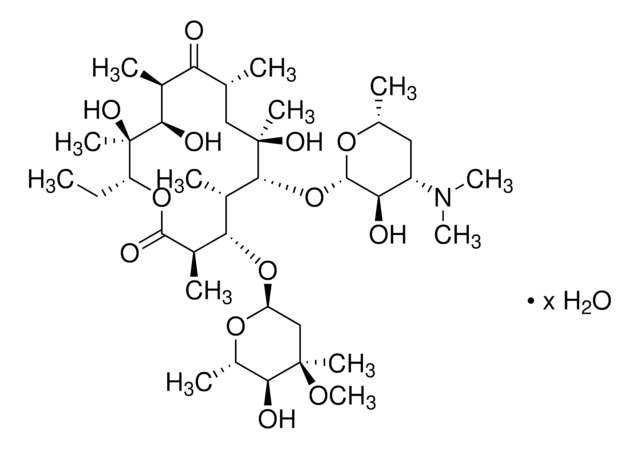
SMILES string
CC[C@H]1OC(=O)[C@H](C)[C@@H](O[C@H]2C[C@@](C)(OC)[C@@H](O)[C@H](C)O2)[C@H](C)[C@@H](O[C@@H]3O[C@H](C)C[C@@H]([C@H]3O)N(C)C)[C@](C)(O)C[C@@H](C)C(=O)[C@H](C)[C@@H](O)[C@]1(C)O
InChI
1S/C37H67NO13/c1-14-25-37(10,45)30(41)20(4)27(39)18(2)16-35(8,44)32(51-34-28(40)24(38(11)12)15-19(3)47-34)21(5)29(22(6)33(43)49-25)50-26-17-36(9,46-13)31(42)23(7)48-26/h18-26,28-32,34,40-42,44-45H,14-17H2,1-13H3/t18-,19-,20+,21+,22-,23+,24+,25-,26+,28-,29+,30-,31+,32-,34+,35-,36-,37-/m1/s1
InChI key
ULGZDMOVFRHVEP-RWJQBGPGSA-N
Gene Information
human ... ABCB1(5243) , CYP3A4(1576) , MLNR(2862)
mouse ... Abcb1a(18671) , Abcb1b(18669)
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
- as a supplement for nutrient broth medium for culturing green fluorescent protein (GFP)- expressing E. coli
- as a model drug to determine small intestinal (SMI) microtissue viability using the MTT assay{254
- as an antibiotic to study the treatment strategies of chronic infections
Biochem/physiol Actions
Antimicrobial Spectrum: This product acts against both gram-negative and gram-positive bacteria.
Caution
Preparation Note
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Protein synthesis is a complex, multi-step process involving many enzymes as well as conformational alignment. However, the majority of antibiotics that block bacterial protein synthesis interfere with the processes at the 30S subunit or 50S subunit of the 70S bacterial ribosome.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico