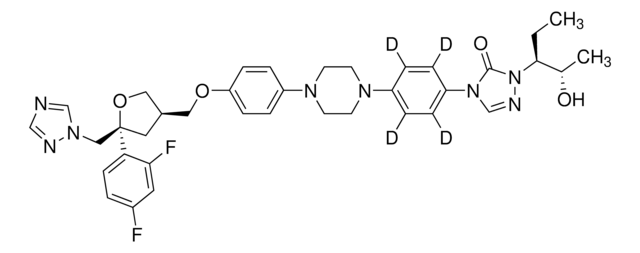
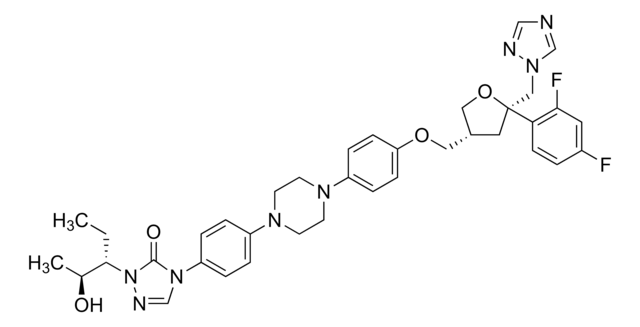
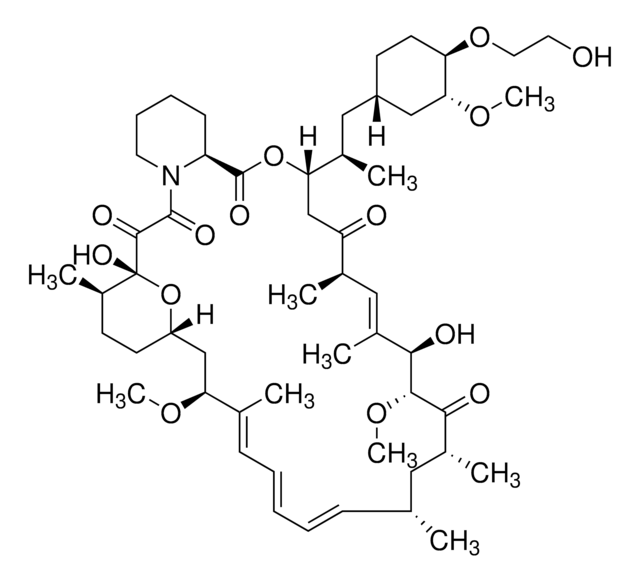
P-103
Posaconazole solution
2.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Productos recomendados
grade
certified reference material
Quality Level
form
liquid
feature
(Snap-N-Spike®)
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
concentration
2.0 mg/mL in methanol
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
clinical testing
format
single component solution
storage temp.
room temp
InChI
1S/C37H42F2N8O4/c1-3-35(26(2)48)47-36(49)46(25-42-47)31-7-5-29(6-8-31)43-14-16-44(17-15-43)30-9-11-32(12-10-30)50-20-27-19-37(51-21-27,22-45-24-40-23-41-45)33-13-4-28(38)18-34(33)39/h4-13,18,23-27,35,48H,3,14-17,19-22H2,1-2H3/t26-,27+,35-,37-/m0/s1
InChI key
RAGOYPUPXAKGKH-XAKZXMRKSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
- Pharmacokinetic modeling in pediatric patients: Posaconazole intravenous solution and oral powder suspension formulations are studied for their pharmacokinetics in pediatric patients with neutropenia, highlighting the solution′s role in optimizing dosing regimens (Winchell et al., 2024).
- Enhanced solubility and bioavailability: Posaconazole antifungal solution is utilized in the development of amorphous solid dispersions with poly(vinylpyridine-co-vinylpyridine N-oxide) excipients, which mediate rapid dissolution and sustained supersaturation, improving its therapeutic efficacy (Liu et al., 2024).
- Nanoformulation for oral delivery: A posaconazole-loaded phospholipid-based nanoformulation developed through design of experiments, machine learning, and TOPSIS optimizes oral delivery, enhancing the antifungal′s absorption and therapeutic profile (Bayat et al., 2024).
- Cost-utility analysis in fungal prophylaxis: Research on posaconazole solution′s cost-effectiveness in preventing invasive fungal infections among leukemia patients undergoing chemotherapy underscores its potential economic benefits in healthcare settings (Pungprasert et al., 2024).
- Cerebrospinal fluid concentrations: Studies assessing the concentrations of posaconazole in cerebrospinal fluid of pediatric leukemia patients validate the solution′s penetration and potential effectiveness in treating central nervous system infections (Körholz et al., 2024).
Legal Information
Related product
signalword
Danger
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
target_organs
Eyes,Central nervous system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 2
flash_point_f
49.5 °F
flash_point_c
9.7 °C
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico