D206008
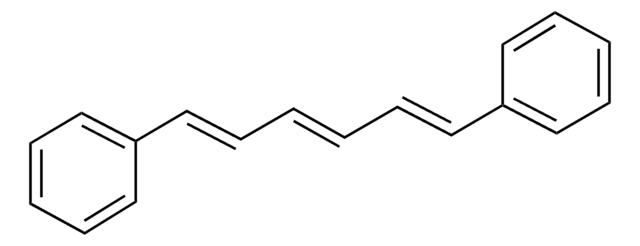
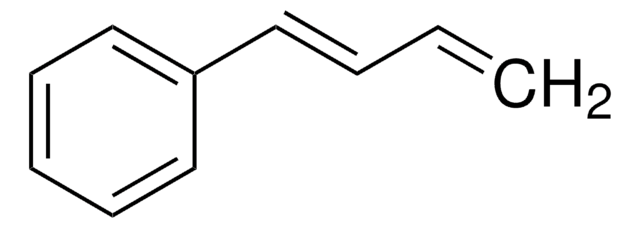
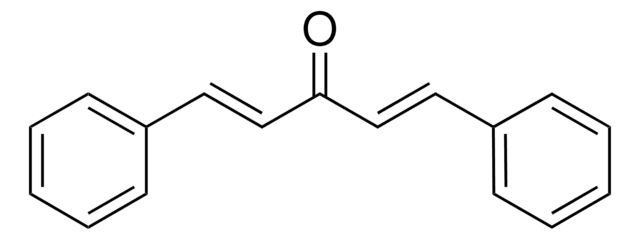
trans,trans-1,4-Diphenyl-1,3-butadiene
98%
Sinónimos:
β,β′-Bistyryl, DPB
About This Item
Productos recomendados
Quality Level
assay
98%
form
crystals
bp
350 °C (lit.)
mp
150-152 °C (lit.)
SMILES string
c1ccc(cc1)\C=C\C=C\c2ccccc2
InChI
1S/C16H14/c1-3-9-15(10-4-1)13-7-8-14-16-11-5-2-6-12-16/h1-14H/b13-7+,14-8+
InChI key
JFLKFZNIIQFQBS-FNCQTZNRSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Application
- 2,5-diphenylthiophene by oxidation reaction with potassium sulfide and DMSO.
- 2-[(3E)-4-Phenyl-2-(phenylmethyl)-3-buten-1-yl]furan via nickel catalyzed hydrobenzylation reaction with furfural in the presence of N2H4.
It can also be used as a ligand to prepare silver(I) coordination polymers by reacting with silver(I) salts.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Global Trade Item Number
| Número de referencia del producto (SKU) | GTIN |
|---|---|
| D206008-25G | 4061833561485 |
| D206008-5G | 4061833317488 |
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico