685879
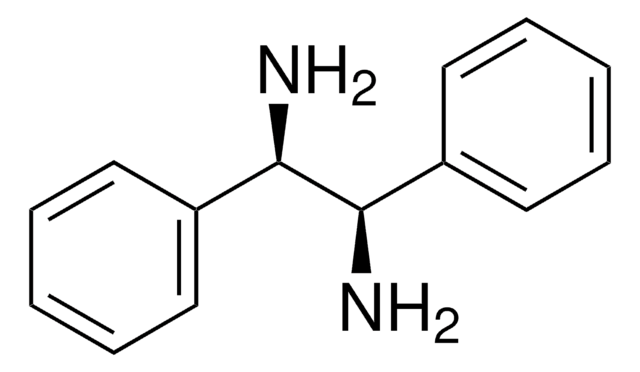
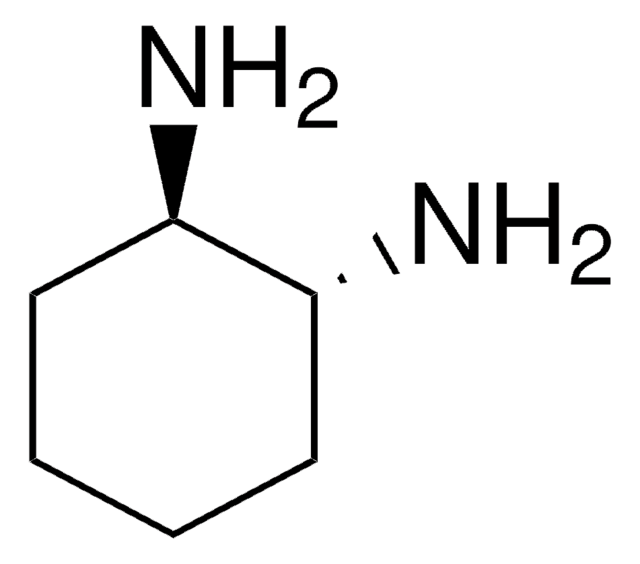
(1S,2S)-1,2-Bis(2-hydroxyphenyl)ethylenediamine
95%
Sinónimos:
(1S,2S)-1,2-Bis(2-hydroxyphenyl)-1,2-ethanediamine
About This Item
Productos recomendados
assay
95%
form
solid
optical activity
[α]22/D -65°, c = 0.2 in chloroform
mp
157-162 °C
SMILES string
N[C@H]([C@@H](N)c1ccccc1O)c2ccccc2O
InChI
1S/C14H16N2O2/c15-13(9-5-1-3-7-11(9)17)14(16)10-6-2-4-8-12(10)18/h1-8,13-14,17-18H,15-16H2/t13-,14-/m0/s1
InChI key
MRNPLGLZBUDMRE-KBPBESRZSA-N
General description
Application
- As a starting material for the synthesis of Schiff base complexes of gold(III), bearing potent anticancer activity.
- As a stereoinductor in the synthesis of quinoline and isoquinoline based 1,2-diamines; that are employed as catalysts in the preparation of warfarin and coumachlor in water.
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Chiral vicinal diamines are of tremendous interest to the synthetic chemist as they are found in many chiral catalysts and pharmaceuticals.
Contenido relacionado
The Chin group is interested in computational and experimental approaches to understanding stereoselective recognition and catalysis. Their studies in weak forces (H-bonding, electronic and steric effects) has led to a highly efficient method for making limitless varieties of chiral vicinal diamines from the 'mother diamine' that are useful for developing stereoselective organocatalysts or transition metal-based catalysts as well as for developing drugs (Acc Chem Res (2012) p1345). The 'mother diamine' is also useful for making binol, monophos and binap analogs. The Chin group is also interested in using reversible covalent bonds for stereoselective recognition and L to D conversion of natural and non-natural amino acids (EJOC (2012) p229).
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico