531464
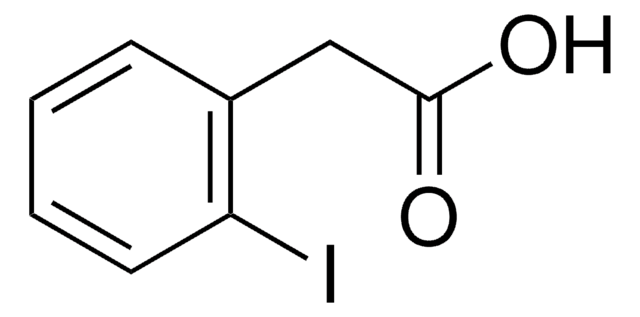
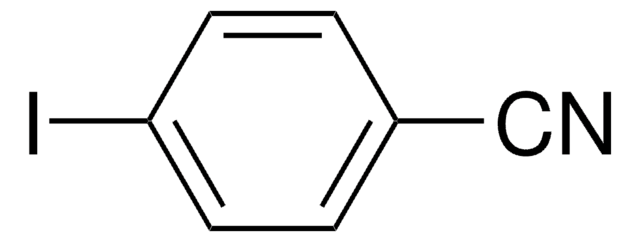
2-Iodophenylacetonitrile
97%
Sinónimos:
2-Iodobenzyl cyanide
About This Item
Productos recomendados
assay
97%
refractive index
n20/D 1.618 (lit.)
bp
113-120 °C/0.5 mmHg (lit.)
density
1.75 g/mL at 25 °C (lit.)
functional group
iodo
nitrile
SMILES string
Ic1ccccc1CC#N
InChI
1S/C8H6IN/c9-8-4-2-1-3-7(8)5-6-10/h1-4H,5H2
InChI key
FPSGTRJUQLYLHE-UHFFFAOYSA-N
General description
Application
- 2?-aminobiphen-2-ylacetonitrile
- ethyl (2-iodophenyl)iminoacetate hydrochloride
- 3,4-disubstituted 2-naphthalenamines
It may also be used in the preparation of the following nitriles:
- 2-(2-iodophenyl)-2-methylpropanenitrile
- 1-(2-iodophenyl)cyclopentanecarbonitrile
- 5-bromo-2-(2-iodophenyl)pentanenitrile
- 2-(2-iodophenyl)-2-propylpentanenitrile
- 1-(2-iodophenyl)cyclohexanecarbonitrile
- 1-(2-Iodophenyl)cyclopropanecarbonitrile
signalword
Warning
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
230.0 °F - closed cup
flash_point_c
110 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico