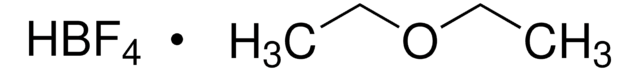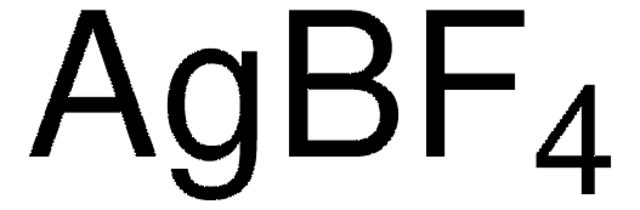482358
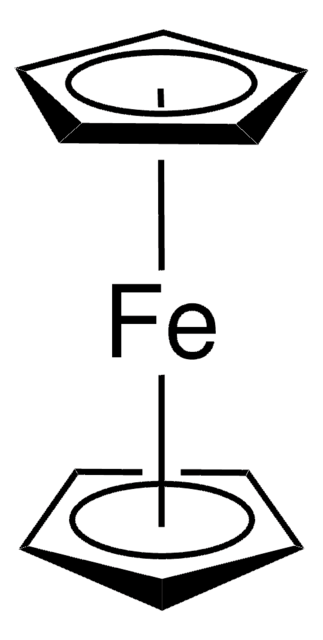
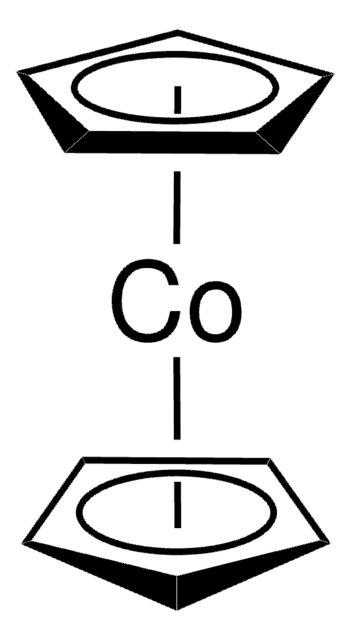
Ferrocenium tetrafluoroborate
technical grade
Sinónimos:
Bis(cyclopentadienyl)iron tetrafluoroborate, Dicyclopentadienyliron fluoborate, Dicyclopentadienyliron tetrafluoroborate
About This Item
Productos recomendados
grade
technical grade
reaction suitability
core: iron
reagent type: catalyst
mp
178 °C (dec.) (lit.)
SMILES string
[Fe+].F[B-](F)(F)F.[CH]1[CH][CH][CH][CH]1.[CH]2[CH][CH][CH][CH]2
InChI
1S/2C5H5.BF4.Fe/c2*1-2-4-5-3-1;2-1(3,4)5;/h2*1-5H;;/q;;-1;+1
InChI key
ZSPXIHLQPWVOQR-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
- an oxidizing agent in the synthesis of the monocationic Co(II)complex [CpCo(azpy)]+
- a Lewis acid catalyst in epoxide ring opening and to activatethe carbonyl group for addition or cycloadditions reactions
- an oxidizing agent when used in conjuntion with a Cl-source
- a reversible redox reagent between stannole dianion and bistannole-1,2-dianion
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico