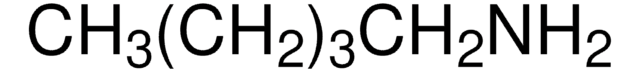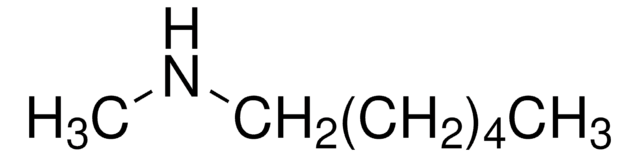219703
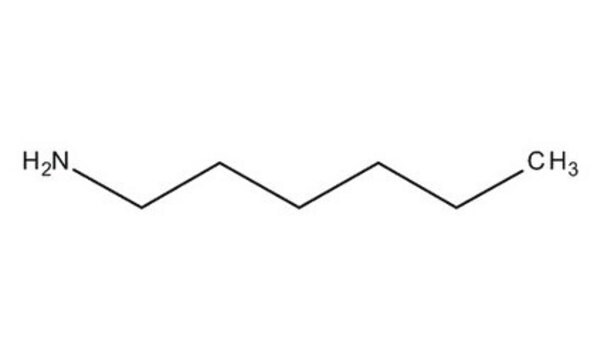
Hexylamine
99%
Sinónimos:
1-Aminohexane
About This Item
Productos recomendados
Quality Level
assay
99%
form
liquid
expl. lim.
2.1-9.3 %
refractive index
n20/D 1.418 (lit.)
bp
131-132 °C (lit.)
mp
−23 °C (lit.)
density
0.766 g/mL at 25 °C (lit.)
functional group
amine
SMILES string
CCCCCCN
InChI
1S/C6H15N/c1-2-3-4-5-6-7/h2-7H2,1H3
InChI key
BMVXCPBXGZKUPN-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Application
- As an initiator to synthesize defined polypeptides by primary amine-initiated N-carboxyanhydride ring opening polymerization reaction.
- As a reactant to modify alkanethiol monolayers at polycrystalline gold surfaces via amide bond formation reaction.
- To functionalize the surface of MWCNT, graphene oxide, and polyurethanes. These functionalized composites materials find applications in absorption, CO2 capture, and as barrier materials.
signalword
Danger
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1A
Storage Class
3 - Flammable liquids
wgk_germany
WGK 1
flash_point_f
80.6 °F - closed cup
flash_point_c
27 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Protocolos
Information on the Amide bond and the Catalytic Amide Bond Formation Protocol. Amidation of amines and alcohols. The amide bond, an important linkage in organic chemistry, is a key functional group in peptides, polymers, and many natural products and pharmaceuticals.
Separation of Propylamine; Butylamine; Pentylamine; Hexylamine; Heptylamine; Octylamine; Nonylamine; Decylamine
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico