XtalFluor-E® and XtalFluor-M®: Convenient, Crystalline Deoxofluorination Reagents
Troy Ryba

Figure 1.Structures of the new deoxofluorination reagents, XtalFluor-E (719439) and XtalFluor-M (719447).
The introduction of fluorine into the framework of an organic molecule is an important synthetic transformation. Historically, though, this transformation has been fraught with technical challenges when using traditional fluorination reagents such DAST and Deoxo-Fluor®. In particular, the chemical practitioner has been forced to weigh the benefits of these once best-in-class reagents against the disadvantages of their stringent handling and safety requirements.
With the recent introduction of XtalFluor-E and XtalFluor-M by Couturier and coworkers, however, many of the issues surrounding the safety and handling requirements of traditional fluorination reagents are no longer of concern.2 XtalFluors, as their name intimates, are crystalline deoxofluorination reagents amenable to short-term handling open to the atmosphere. The substrate and reactivity profiles of the XtalFluors are also comparable to the traditional deoxofluorination reagents (Scheme 1): alcohols are converted to the corresponding alkyl fluorides, aldehydes and ketones to the geminal-difluorides, carboxylic acids to the acyl fluorides, sulfoxides to the fluoromethyl thioethers and hemiacetal sugars to the corresponding glycosyl fluoride donors.

Scheme 1.Generic substrate scope of XtalFluor-mediated deoxofluorination.
An intrinsic property of the XtalFluors is that they do not generate free HF under anhydrous reaction conditions. While the mechanism is still under investigation, it is thought that the XtalFluors activate the C–O bond without concomitant fluoride release. Only upon subsequent exposure to a promoter such as DBU, Et3N ∙ 3HF, or Et3N ∙ 2HF, does fluoride attack the activated carbon atom. The chemical efficiency of the deoxofluorination is high.
The stability of the XtalFluors has also been investigated by Accelerated Rate Calorimetry (ARC). These studies have shown that the XtalFluors have greater thermal stability than DAST or Deoxo- Fluor (Table 1). This may be of particular practical interest when considering reagents to utilize for scale-up routes.
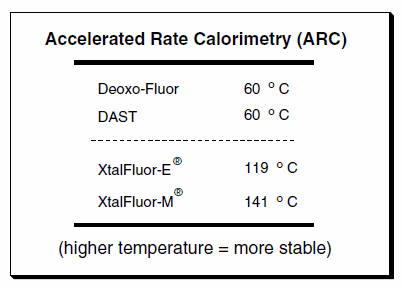
Table 1.Accelerated Rate Calorimetry (ARC) data comparisons indicating a higher thermal stability of the XtalFluors.
References
To continue reading please sign in or create an account.
Don't Have An Account?