Protocol Guide: Expansion and Differentiation of Human Mesenchymal Stem Cells using TrueGel3D® HTS Hydrogel Plates
Recently, 3D cell culture disease models of have become attractive alternatives to animal models and traditional 2D cell cultures. 3D cultured human mesenchymal stem cells (MSCs) have been proven to become more therapeutically functional leading to enhanced clinical outcomes. Additionally, co-cultures of MSCs with stromal cells, such as fibroblast, have shown to produce more physiologically relevant cellular phenotypes. However, these models often rely on undefined animal derived hydrogels which are not amendable to high throughput screening applications.
The TrueGel3D® HTS Hydrogel Plate is a ready-to-use solution to easily establish 3D cell cultures using fully synthetic hydrogels in a simple and automation-compatible manner. Here we demonstrate how human adipose (HA) derived stem cells can be expanded and differentiated into adipocytes or osteocytes using TrueGel3D® HTS hydrogel plates following an optimized step-by-step protocol.
Mesenchymal Stem Cell Protocol
Expansion and Differentiation of Human MSCs
Human adipose mesenchymal stem cells (SCC038) can be expanded on tissue culture plastic first and then seeded in TrueGel3D® HTS Hydrogel Plate (TRUE-HTS1, TRUE-HTS10). Alternatively, cells can be thawed and directly seeded in TrueGel3D® HTS Hydrogel Plate. Ensure cells are at high viability before seeding into TrueGel3D® HTS hydrogel plates. The cells should be handled and stored according to the manufacturer’s instructions. All steps should be performed inside a laminar flow hood to ensure sterility.
- Preparation of culture media:
- 2D MSC Expansion Media: Supplement α-MEM (M4526) with 10% FBS (ES-020-B), 1x Glutamine (TMS-002), and 1x Pen/Strep (P4333). Add 5 ng/ml FGF (GF003AF) to the medium prior to usage (aliquots of medium containing FGF can be stored at 4°C for up to one week, avoid cool-warm cycle).
- 3D MSC Expansion Media: Supplement α-MEM (M4526) with 10% FBS (ES-020-B), 1x Glutamine (TMS-002), and 1x Pen/Strep (P4333). Add 50 ng/ml FGF (GF003AF) and 50 ng/ml PDGF (P3201) to the medium prior to usage (aliquots of medium containing FGF/PDGF can be stored at 4°C for up to one week, avoid cool-warm cycle).
- Adipogenesis Differentiation Media: Supplement DMEM (SLM021) with 20% FBS (ES-020-B), 1x Glutamine (TMS-002), 1x Pen/Strep (P4333), 10 µg/mL insulin (I2643), 0.5 mM 3-Isobutyl-1-methylxanthin (I5879), 0.1 mM Indomethacin (I7378) and 1μM Dexamethasone (D4902).
- Osteogenesis Differentiation Media: Supplement α-MEM (M4526) with 10% FBS (ES-020-B), 1x Glutamine (TMS-002), 1x Pen/Strep (P4333), 50 μg/mL L-ascorbic acid (A4403), 10 mM β-glycerol phosphate (G9422) and 100 ng/mL Bone morphogenetic protein 2 (BMP-2) (SRP3326).
- Add 200 µl/well of the human adipose MSC suspension in 3D expansion media to the TrueGel3D® HTS hydrogel plate at a density of 30,000 cells/well. Note: cell density can be adjusted according to specific assay parameters, e.g. days of culture.
- Change medium every 2-3 days. Note: Track the culture development using a transmission light microscope. Immediately after seeding, HA-MSCs will appear round on top of the hydrogel, but within approximately 24 hours they will start to invade the hydrogel forming a 3D network as shown in the following section.
- To induce differentiation, change media to osteogenic or adipogenic differentiation media and monitor over 14-21 days exchanging media every 2-3 days. Note: After 14-21 days of differentiation the culture will contain Oil-Red-O positive (adipocytes) or alkaline phosphatase ALP positive cells (osteocytes).
Antibody Characterization by Microscopy
- Aspirate the culture medium.
- Fix the cells with 4% formaldehyde solution (1.00496) for 30 min at room temperature.
- Wash three times with PBS (D8537) (200 µl/well) for 5-10 min each. Note: the plates can be closed again with the sealing foil and stored at 4 °C for future use
- Block for 2 hours at room temperature using:
- Incubate overnight at 4°C with 150 µl/well of the appropriate primary antibody diluted accordingly in the corresponding blocking buffer. For example: anti-Collagen Type I (Col-I) antibody (AB745) or anti-Fibronectin (FN) antibody (F0916) can be used at a 1:50 dilution in non-permeable blocking buffer.
- Wash three times with PBS (D8537) (200 µl/well) for 5-10 min each.
- Incubate for 2 hours at room temperature with 200 µl/well of the appropriate fluorescently labelled secondary antibody diluted accordingly in the same blocking buffer. For example: Atto 488 anti-rabbit IgG (62197) or Atto 594 anti-rabbit IgG (76085) can be used at a 1:200 dilution in non-permeable blocking buffer. Note: Hoechst 3358 (94403) counterstaining (1:500 dilution in PBS) can be added together with the secondary antibody to counterstain the cell nuclei (150 µl/well).
- Wash three times with PBS (D8537) (200 µl/well) for 5-10 min each.
- Image: Bright field and fluorescence images showing the neural network spanning the entire depth of the hydrogel can be acquired by an inverted epifluorescence or confocal fluorescence microscope equipped with a 20–30X objective.
Oil-Red-O and Alkaline Phosphatase Staining of Differentiated MSCs
Adipocyte cell differentiation was analyzed using Oil-Red-O staining (1.05230). Osteocyte cell differentiation was analyzed using ALP staining using SIGMAFAST BCIP/NBT tablets (B5655). After 7 days of culture, differentiation medium was removed from cell culture wells and the samples were washed with PBS. BCIP/NBT was then added. After 7 min, the samples were washed with phosphate buffered saline (D8537) once and hydrogels were fixed by 4% paraformaldehyde (PFA) (100496) for 30 min at RT. Finally, samples were rinsed once with PBS and observed under an inversed microscope (Axiovert 200M, Carl Zeiss).
Results
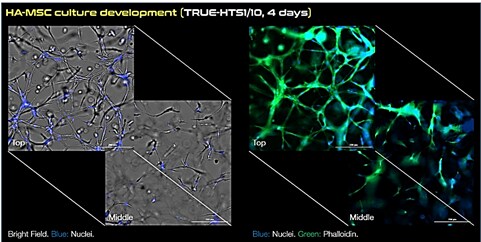
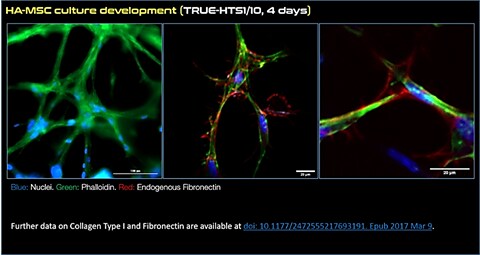

Figure 1.Expansion of human MSCs using TrueGel3D® HTS Hydrogel Plates. Fluorescent staining of day 4 human adipose MSCs cultured in 3D using TrueGel3D® HTS hydrogel plates acquired with an Olympus SpinSR10 spinning disk confocal microscope. MSCs express collagen, fibronectin and display a complex 3D morphology.
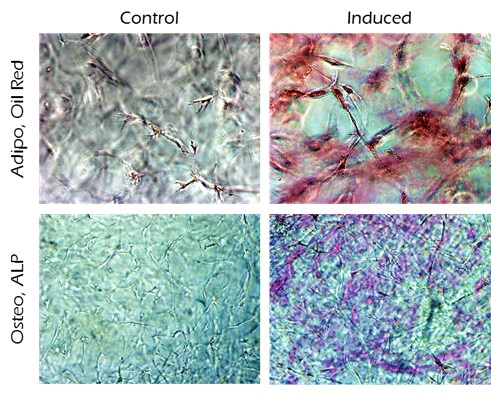
Figure 2.Differentiation of human MSCs using TrueGel3D® HTS Hydrogel Plates. Oil-Red-O and ALP staining of day 14 human adipose MSCs cultured in 3D using TrueGel3D® HTS hydrogel plates acquired with an Olympus SpinSR10 spinning disk confocal microscope.
To continue reading please sign in or create an account.
Don't Have An Account?