Screening for Selectivity of HIC Medium
Figure 19.HiTrap® HIC Selection Kit and RESOURCE™ HIC Test Kit.
Time and sample can be saved in the early stages of development by using small (1 ml), prepacked columns such as those in the HiTrap® HIC Selection Kit and RESOURCE™ HIC Test Kit. Media can be quickly and efficiently screened for the required selectivity. This approach is helpful since, even if the properties of the target protein(s) are known, the final selectivity, binding capacity and recovery depends largely upon the interaction of the medium with the specific protein of interest.
HiTrap® columns are prepacked with hydrophobic interaction media based on Sepharose® High Performance and Sepharose® Fast Flow while RESOURCE™ HIC columns contain media based on SOURCE™. All columns can be used for small-scale purification and are supplied with detailed protocols for use. The media in these test kits are available for large-scale production so that optimized methods can be easily transferred to the required scale of operation.
- Before starting any HIC separation, establish the “salt stability window” for the sample. For example, add increasing amounts of salt to the crude sample in order to establish the concentration at which precipitation occurs. Ensure that the sample is below this salt concentration when applied to a column in order to avoid precipitation. Refer to Appendix 1 for a guide to using ammonium sulfate for sample clean-up by precipitation. When possible, test for biological activity of the target protein to establish the concentration range over which activity can be maintained (remember that the sample may need to be desalted before an activity test).
Automated HIC media screening, method development and optimization
Users of ÄKTAdesign™ chromatography systems can select suitable method templates and program the system to automatically perform separations using a range of columns and a range of buffer conditions.
- Sample preparation: having established the salt stability window, begin with the highest salt concentration that retains biological activity and does not cause precipitation problems. Adjust the sample to the salt concentration of the start buffer to promote hydrophobic interaction. Add salt from a high concentration stock solution to avoid the risk of precipitation due to high salt concentrations that may occur locally if salt is added as a solid. Adjust the pH of the sample directly. Since HIC is not very sensitive to pH conditions, a complete buffer exchange is unnecessary.
- Prepare start and elution buffers: 50 mM phosphate, pH 7.0. Add ammonium sulfate (up to 2 M, according to salt stability window) to the start buffer.
- Wash each column in salt-free elution buffer before equilibrating in a high-salt start buffer. This avoids the risk of salt precipitation when columns have been stored in 20% ethanol.
- Scout for optimum selectivity, applying sample to each column under a range of salt concentrations. Collect eluate throughout the run. Choose a medium in which the target protein(s) elute within the gradient.
- Scout for the lowest salt concentration in the start buffer that maximizes binding capacity for the target protein, maintains or improves resolution and minimizes the risk of contamination from other bound proteins.
- Optimize for the steepest gradient that gives acceptable resolution.
- Optimize for the highest flow rate that maintains resolution and minimizes separation time. Check recommended flow rates for the specific medium.
- Optimize for the maximum sample load that can be applied while maintaining satisfactory resolution. In general, loading 20–30% of the total binding capacity of the column gives optimal resolution with gradient elution.
- Reduce separation time and buffer consumption by transferring to a step elution when optimized separation conditions have been established. Sample loads can often be increased when using a step elution.
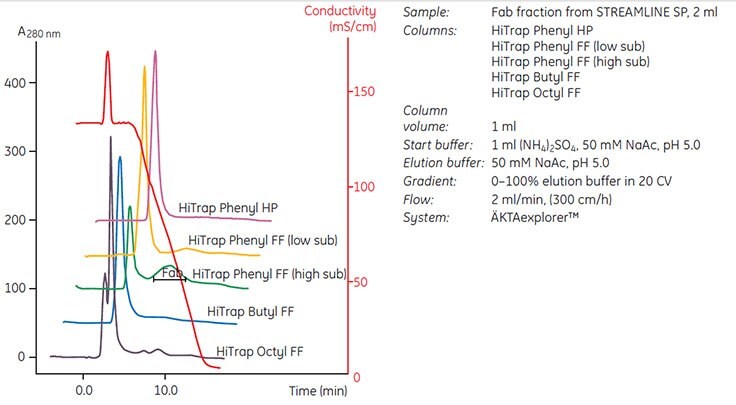
Figure 20. shows an example of media screening on different HIC media prepacked in HiTrap® 1 ml columns.
Buffer pH was kept at pH 5.0 to minimize the need for sample conditioning after capture. Phenyl Sepharose® 6 Fast Flow (high sub) was selected since the medium showed excellent selectivity for the target protein. The protein was eluted within the gradient and separated from the bulk contaminants. Conditions were then optimized so that a step elution could be used to maximize throughput and concentration of the target protein before scaling up. Figure 47 on page 74 shows the optimized elution scheme and subsequent scale-up.
Manual media screening, method development and optimization
HiTrap® columns can be used with a syringe or peristaltic pump for manual media screening, method development and method optimization. However, using a syringe limits the degree to which a HIC separation can be developed since the separation mechanism is not a simple “on/off” process and requires some degree of gradient elution to achieve a satisfactory separation.
The methods here are optimized for use with 1 mL HiTrap® columns and should be adjusted if other column volumes are used. Note that flow rates may need to be reduced due to the viscosity of sample or buffers.
Screening for selectivity using HiTrap® HIC Selection Kit
- Sample preparation: having established the salt stability window, begin with the highest salt concentration that retains biological activity and does not cause precipitation problems. Adjust the sample to the salt concentration of the start buffer to promote hydrophobic interaction.
Add salt from a high concentration stock solution to avoid the risk of precipitation due to high salt concentrations that may occur locally if salt is added as a solid. Adjust the pH of the sample directly. Since HIC is not very sensitive to pH conditions, a complete buffer exchange is not necessary. - Prepare start and elution buffers: 50 mM phosphate, pH 7.0. Add ammonium sulfate (up to 2 M, according to salt stability window) to the start buffer.
- Wash the column(s) with 5–10 mL salt-free elution buffer at 1 mL/min. This avoids the risk of salt precipitation when columns have been stored in 20% ethanol.
- Equilibrate the column(s) with 5–10 mL start buffer at 1 mL/min.
- Apply a known amount of the sample at 1 mL/min. Collect eluate.
- Wash at 1 mL/min with at least 5 mL of start buffer or until no material appears in the eluent. Collect eluate.
- Elute bound material with elution buffer at 1 mL/min (3–5 mL is usually sufficient, but other volumes may be required dependent on the exact experimental conditions). Collect eluate.
- Analyze all eluates (e.g., by an activity assay) and determine purity and the amount bound to the column.
- Select the medium to which the target protein(s) binds and can be eluted. If running a gradient, select the medium that gives the best selectivity and resolution.
Screening for binding (salt) conditions
- Using the selected medium and buffer from the previous protocol, set up a series of start buffers at the same pH, but with reduced concentrations of ammonium sulfate in each buffer (e.g., from 1.5 M, 1.0 M, 0.5 M with a lowest concentration of 0.05 M).
- Repeat steps 2–7 from the previous protocol for each salt concentration.
- Determine the salt concentration that permits binding of the target protein(s) while contaminants either wash through or remain bound to the column. Determine the lowest salt concentration required to achieve complete elution of the target protein.
Optimization of gradient, flow rate and sample loading
- If gradient making equipment is available, determine the steepest gradient that gives acceptable resolution.
- Begin with a gradient of 10 column volumes. Start from the salt concentration required to bind the target protein and go down to the lowest salt concentration required for elution, based on the values determined when screening. Alternatively, begin with a gradient of 0–50% elution buffer and a gradient volume of 10–20 column volumes.
- To save time, determine the highest flow rate that maintains resolution and minimizes separation time. Check recommended flow rates for the specific medium.
- Determine the maximum sample load that can be applied while maintaining satisfactory resolution. In general, loading 20–30% of the total binding capacity of the column gives optimal resolution with gradient elution. Sample loads can often be increased if resolution is satisfactory or when using a step elution.
- Reduce separation time and buffer consumption by transferring to a step elution when optimized separation conditions have been established. Sample loads can often be increased when using a step elution.
Sample properties and choice of ligand
The type of ligand and the nature of the target protein are highly significant parameters in determining the selectivity of a HIC medium. Consequently, the most suitable ligand must be determined empirically through screening experiments (see Screening for selectivity, page 28), preferably using the target protein. Proteins that could be assumed to have very similar properties can interact quite differently under identical experimental conditions in a HIC separation, as demonstrated by the behavior of three monoclonal antibodies in Figure 21.
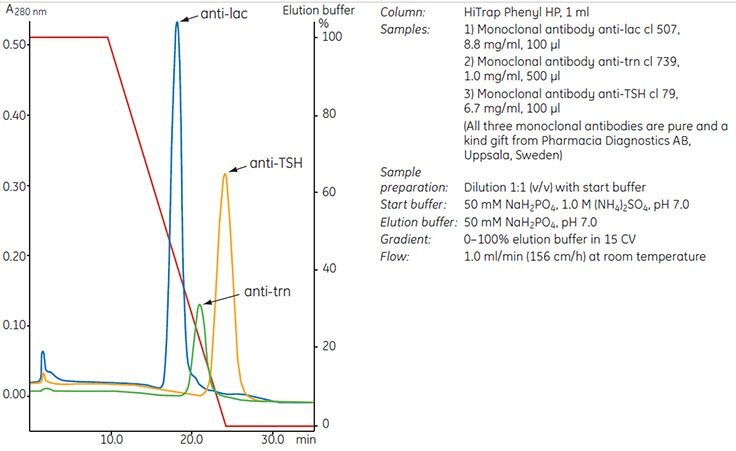
Figure 21.Three monoclonal antibodies interact differently under identical running conditions using a phenyl ligand. A suitable ligand must be determined empirically.
To continue reading please sign in or create an account.
Don't Have An Account?