A Review of High-Sensitivity Immunoassays in PK/PD Studies
- Why Are High-Sensitivity Immunoassays Needed in PK/PD Profiling?
- Ultrasensitive SMC® Immunoassays for PK/PD Studies
- Case Studies
- Benefits of Ultrasensitivity for PK/PD
- References
High-sensitivity immunoassays are critical in pharmacokinetics (PK) and pharmacodynamics (PD) studies to measure low concentrations of drugs. Explore this review of high-sensitivity immunoassays in PK/PD studies to see different case studies showing how ultrasensitive immunoassay technology is used for PK/PD profiling.
Why Are High-Sensitivity Immunoassays Needed in PK/PD Profiling?
Traditional methodologies offer limited capacity for PK/PD profiling and are often unable to show the full clearance profile of some drugs. In addition, they have limited ability to measure the low concentrations of a given drug such as used in micro-dosing studies. High-sensitivity immunoassays have allowed for the detection of lower levels of a drug to provide a more complete PK/PD profile. They also allow for the identification of unpredictable clearance patterns that are undetectable by traditional immunoassay methodologies.
Ultrasensitive SMC® Immunoassays for PK/PD Studies
Single Molecule Counting (SMC®) high-sensitivity immunoassay products and services for PK/PD studies accelerate pharmaceutical, clinical, and life science research. The patented SMCxPRO® immunoassay system is an innovative digital technology that measures single molecules at femtogram per mL concentrations. Using this platform, a broad range of ultrasensitive immunoassays enable the measurement of baseline biomarker concentrations in healthy subjects and differentiation of response to therapy.
Case Studies
See how high-sensitivity SMC® immunoassays were used for PK/PD profiling in these case studies.
Evaluating IL-13 Clearance in Conjunction with Anti-IL-13 Therapeutic Challenges
The situation:
- IL-13 is a Th2 cytokine implicated in asthmatic inflammation
- Anti-IL-13 therapeutics are being developed to counter autoimmune diseases
- Accurate measurement of circulating IL-13 levels is needed for PK/PD and mode of action (MOA) studies
The problem:
- IL-13 quantitation requires improved sensitivity over traditional immunoassay or ELISA techniques (circulating levels ≤ 1 pg/mL)
The solution:
- Quantify IL-13 with improved assay sensitivity1
- SMC® assays enabled quantification of baseline measurements of a clinical study population (n=182 from 3 cohorts)
- All 99th% cut-off values for healthy and asthmatic subjects at baseline were less than 1 pg/mL meaning they were not measurable by the ELISA method
- Monitor pre- and post-dosing of anti-IL-13 mAbs in subjects with mild atopic stable asthma2
- Antibodies IMA-026 and IMA-638 were evaluated for anti-IL-13 treatment. Each competes with different receptors for IL-13 binding.
- SMC® IL-13 assay (LLOQ = 0.07 pg/mL) enabled longitudinal measurement of serum IL-13 levels from antibody and placebo-treated subjects (Table 1)
- ELISA (LLOQ = 9.8 pg/mL) would be unable to measure the full profile for antibody- or placebo-treated subjects (Figure 1)
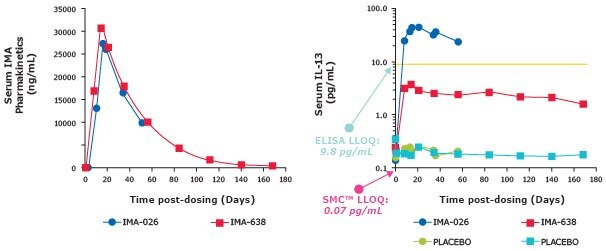
Figure 1.Pharmacokinetic graphs showing SMC® assays have a lower LLOQ than ELISAs.
Complete PK Profile of Serum cTnl in Rat and Dog3
The situation:
- The effect of minor serum cardiac troponin I (cTnI) elevations, independent of extensive cardiomyocyte damage, has not been evaluated thoroughly because traditional assays have not been sensitive enough for this purpose (LLOQ ~30 pg/mL)
- Transient or slight cardiomyocyte damage may not generate a large and persistent release of cTnI, making it difficult to identify a treatment-related transient increase using limited sampling times
The problem:
- Evidence suggests that small elevations of serum cTnI above baseline are correlated with an increased risk of myocardial-related mortality in humans
- The ability to establish and monitor baseline concentrations of serum cTnI in rat or dog is essential to assessing the cardiovascular safety of therapeutic compounds in development
The solution:
- Single digit pg/mL baseline concentrations of cTnI were detected using the SMC® Human cTnI High Sensitivity Kit (when previously undetectable by other cTnI assays)
- Biphasic disposition of serum cTnI was observed after intravenous (IV) injection
- The complete cTnI profile was exposed using select low dose cTnI concentrations estimated to be equivalent to cTnI levels expected after slight cardiomyocyte damage
- cTnI levels fall below LLOQ for previous assays within 1 hour of dosing
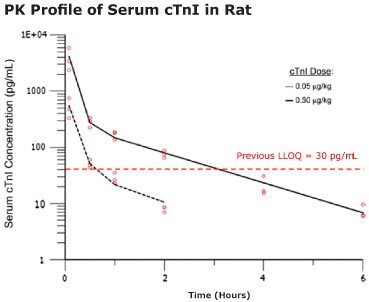
Figure 2.The pharmacokinetics of cTnI in the Wistar Han rat was analyzed with a two-compartment model. 300 μL rat blood samples were collected at multiple time points and measured for cTnI. Open circles are serum cTnI concentrations from individual animals.
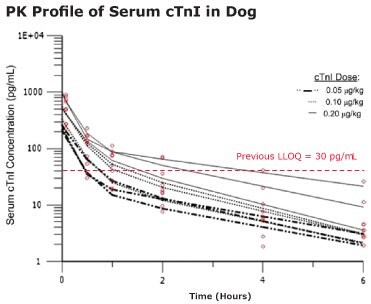
Figure 3.The pharmacokinetics of cTnI in the beagle dog were analyzed with a two-compartment model. Open circles are serum cTnI concentrations from individual animals. Each data line tracks the serum cTnI concentration from the same dog post-dosing. Values are reported as means and SDs.
Benefits of Ultrasensitivity for PK/PD
SMC® high-definition ultrasensitive immunoassays provide new perspectives for low therapeutic index drug development.
Benefits of this ultrasensitivity include:
- High-value PK/PD data at low doses
- Optimized therapeutic index at low doses (Figure 4)
- Clear understanding of safety and efficacy
- Informed go/no-go decisions in Phase I/II trials
- Reduced attrition and development costs
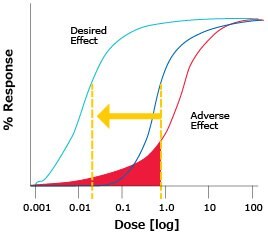
Figure 4.Ultrasensitive measurement enables you to detect small differences in the minimum effective concentration and minimum toxic concentration of a drug with a narrow therapeutic index.
The SMC® ultrasensitive immunoassay platform can detect much lower levels of drug ensuring that relevant biology can be discovered. This is important for micro-dosing studies since they can be below the level of detection by conventional PK methodologies (Figure 5).

Figure 5.Example of a micro-dosing study showing the need for a lower level of detection to get the full PK profile. Work completed at Singulex® Inc., Alameda, California. 2013.
With years of extensive experience sample testing, our in-house SMC® custom assay development group can assist you in your immunogenicity assay development, including cut point analysis, customer ADA sample testing, PK/PD analysis, and full kit manufacture (ISO 9001).
Learn more about our comprehensive portfolio of homebrew kits and reagents for developing the assay that suits your specific drug-development application.
References
To continue reading please sign in or create an account.
Don't Have An Account?