MILLIPLEX® Human Cytokine/Chemokine/Growth Factor Panel A: Larger and Customizable Cytokine Multiplex Assay
Cytokine multiplex assays allow researchers to easily investigate the immune system and inflammation mechanisms. The MILLIPLEX® Human Cytokine/Chemokine/Growth Factor Panel A is a cytokine multiplex assay that combines tests for 48 individual immune factors that previously have not been together in a single panel, while also allowing you to choose any combination of analytes, or select a 48-plex or a 38-plex premixed kit. Read on to see how this panel was built with quality in mind and has improved workflow and sample detection that help you discover with confidence.
Role of Cytokines, Chemokines, and Growth Factors
Cytokines mediate direct interactions between cells and regulate processes taking place in the extracellular environment. Cytokines differ from hormones in that they act on a wider spectrum of target cells and include lymphokines, interferons, colony-stimulating factors, and chemokines. Additionally, growth factors are involved in the stimulation of target cell survival, proliferation, and differentiation with effects on angiogenesis, vasculogenesis, and cell migration.
Cytokine, chemokine, and growth factor research plays a significant role in achieving a deeper understanding of the immune system and its multi-faceted response to most antigens. This deep understanding is especially relevant to those responses that make up the inflammatory process and disease states, such as osteoarthritis, IBD, sepsis, allergic reactions, and even cancer. It also allows researchers to understand infectious, respiratory, neurologic, metabolic, and cardiovascular diseases.
Cytokine multiplex assays allow researchers to measure multiple cytokines, chemokines, and growth factors from one small sample, saving time and resources. For example, MILLIPLEX® multiplex assays based on Luminex® xMAP® technology offer researchers a variety of cytokine panels to dive deeper into immune and inflammatory processes. Explore how multiplex assays can help to understand cytokine storm in our MILLIPLEX® Multiplex Assays for Research Applications in COVID-19 (SARS-CoV-2) article.
For Research Use Only. Not For Use In Diagnostic Procedures.
Quality Built into MILLIPLEX® Kits
Kit development and verification entails testing for selectivity and specificity to ensure negligible cross-reactivity in the tested sample types, as well as assay specificity to ensure consistent performance of an assay in single-plex vs. multiplex formats. Buffers and diluents are optimized to enhance antibody specificity, such that only those analytes of interest are detected in samples. The serum matrix is also carefully selected and optimized for use in the standard curve when using serum or plasma samples to most closely mimic sample matrix, thus normalizing assay performance. The streptavidin-phycoerythrin (SAPE) concentration is titrated in-house for optimal signal and is provided ready-to-use with no dilution required. Additionally, all our kits are rigorously tested for shipping stability, and samples are also tested for temperature and freeze/thaw tolerance.
Sample dilution is optimized for each analyte in the kit, ensuring that biologically relevant sample values are detectable, and fall within the dynamic range of the standard curves. Samples tested for this kit include normal and disease serum and plasma samples, as well as peripheral blood mononuclear cell supernatants (PBMCs, unstimulated and stimulated with various agents). Quality Controls (QCs), which are low and high dilutions of recombinant proteins for each analyte, are manufactured such that the two QCs (high and low) have optimal placement on each standard curve. QC range sheets are provided with each kit. Additionally, we always recommend users include experiment-specific samples for use as controls in each assay.
Learn more in our article about quality of MILLIPLEX® kits.
Workflow Improvements
An assay containing 48 individual immune factors in a single panel is a powerful tool for research. The ability to select all analytes, perhaps for screening, and then to select a subset of analytes as a project continues, allows researchers the flexibility they need to work efficiently. Detecting all (or a subset) of these proteins at pg/mL levels within a kit with standard curves that do not change from lot to lot makes the assay very user-friendly. Consistent standard curves from lot to lot ensure consistent sample results across a project (Figure 1).
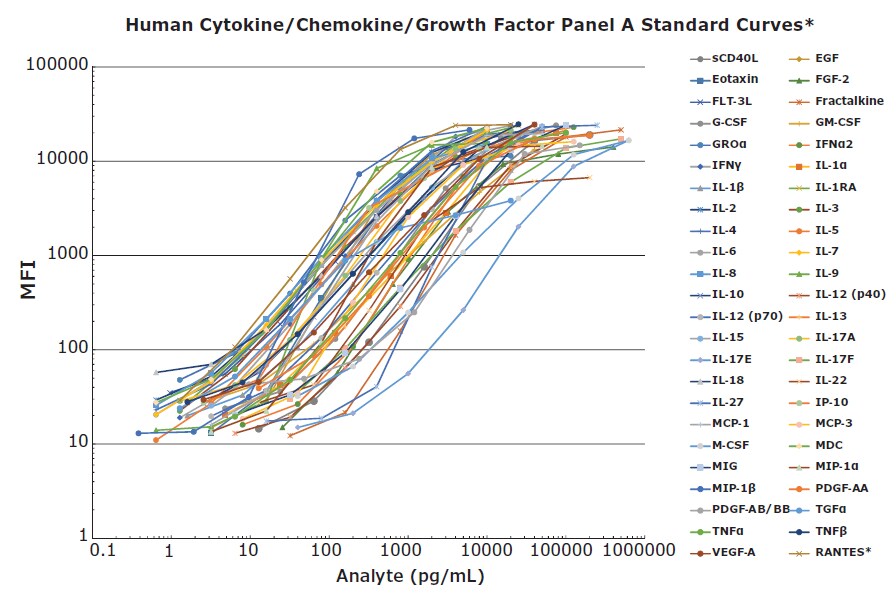
Figure 1.48-plex standard curves were performed in the MXHSM-A serum matrix except *RANTES, which was performed in the L-AB assay buffer. The kit was run using the MILLIPLEX® Human Cytokine/Chemokine/Growth Factor Panel A overnight assay protocol.
The MILLIPLEX® Human Cytokine/Chemokine/Growth Factor Panel A also has a straightforward protocol with a familiar workflow in which every step is outlined to ensure ease of use.
Another assay improvement is that both types of platelet-derived growth factor PDGF-AA and PDGF-AB/BB can now be tested with neat serum and plasma samples, so there is no need for sample dilution as is the case with MILLIPLEX® Human Cytokine/Chemokine Panel 1, or for other Luminex® - based kits on the market. RANTES, being highly expressed in serum and plasma samples, still requires a separate sample dilution in serum and plasma of 1:100.
A simple assay improvement of logically arranging the bead regions and analyte names in numeric-alphabetic order, allows researchers to quickly locate specific analytes in the result output files much easier than with previous assays. This helps immensely when analyzing datasets of 42 analytes or greater.
Sample Detectability
In addition to improvements in the standard curves relative to the comparative analyte assays for the MILLIPLEX® Human Cytokine/Chemokine/Growth Factor Panel A, there is also improvement in analyte performance in terms of sample detection. One goal in the development of this panel was to closely match sample values when evaluated against comparison MILLIPLEX® kits (Cat. No. HCYTOMAG-60K, HCYP3MAG-63K, or HTH17MAG-14K). Of note, three of the analyte assays have been adjusted to better match the accepted values in the literature: GROα1,2, IL-41, and IL-223,4,5.
Figures 2 and 3 show how this panel was used for analyzing disease samples and stimulated PBMCs respectively.

Figure 2.Healthy control serum/plasma samples (obtained from BioIVT) and sepsis patient serum/plasma samples (obtained from BioIVT, Discovery, and BioChemed) were tested neat (25 μL/well) in the MILLIPLEX® Human Cytokine/Chemokine/Growth Factor Panel A. Healthy control serum/plasma samples, N=20. Sepsis serum/plasma samples, N=16.

Figure 3.Human PBMC samples (obtained from BioIVT) in RPMI containing 10% FBS and 1% Penicillin/ Streptomycin at 106 cells/mL were incubated with either 1 μg/mL LPS or Con A for 48 hours at 37 °C, after which cell-free supernatants were collected and tested with the MILLIPLEX® Human Cytokine/Chemokine/Growth Factor Panel A.
More details can be found in our MILLIPLEX® Human Cytokine/Chemokine/Growth Factor Panel A application note.
For Research Use Only. Not For Use In Diagnostic Procedures.
Recent Publications Using MILLIPLEX® Human Cytokine/Chemokine/Growth Factor Panel A
- Epigenetic state determines inflammatory sensing in neuroblastoma.
Wolpaw AJ, Grossmann LD, Dessau JL, Dong MM, Aaron BJ, Brafford PA, Volgina D, Pascual-Pasto G, Rodriguez-Garcia A, Uzun Y, et al. 2022. Proc. Natl. Acad. Sci. U.S.A.. 119(6): https://doi.org/10.1073/pnas.2102358119. - Regulatory T Cells Control Effector T Cell Inflammation in Human Prediabetes. Liu R, Pugh GH, Tevonian E, Thompson K, Lauffenburger DA, Kern PA, Nikolajczyk BS. 2022. 71(2):264-274. https://doi.org/10.2337/db21-0659.
- Inhibition of tumor necrosis factor improves conventional steroid therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis in a cohort of patients. Ao S, Gao X, Zhan J, Ai L, Li M, Su H, Tang X, Chu C, Han J, Wang F. 2022. Journal of the American Academy of Dermatology. https://doi.org/10.1016/j.jaad.2022.01.039.
References
To continue reading please sign in or create an account.
Don't Have An Account?