1A00030
USP
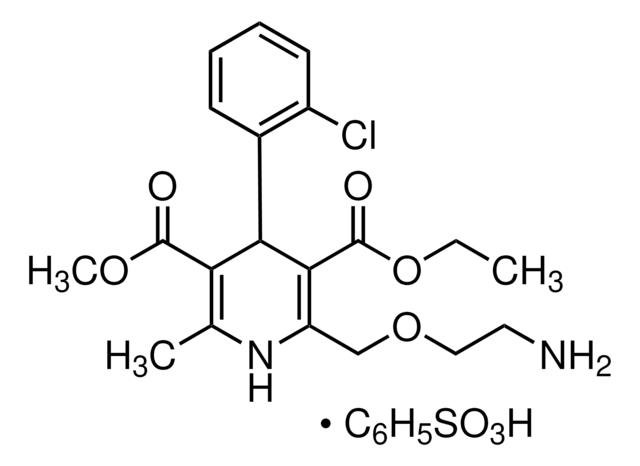
Amlodipine Methyl Analog
Pharmaceutical Analytical Impurity (PAI)
同義詞:
(dimethyl 2-((2-aminoethoxy)methyl)-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate, maleate)
登入查看組織和合約定價
全部照片(1)
About This Item
經驗公式(希爾表示法):
C19H23ClN2O5·C4H4O4
CAS號碼:
分子量::
510.92
MDL號碼:
分類程式碼代碼:
41116100
NACRES:
NA.24
推薦產品
等級
pharmaceutical analytical impurity (PAI)
agency
USP
API 家族
amlodipine
製造商/商標名
USP
應用
pharmaceutical
形式
neat
儲存溫度
2-8°C
一般說明
Amlodipine Methyl Analog is a USP Pharmaceutical Analytical Impurity (PAI).
USP PAI are a product line of impurities suitable for research and analytical purposes, which help to ensure the quality and safety of medicines.
Associated Drug Substance: Amlodipine
Therapeutic Area: Cardiovascular.
For more information about this PAI, visit here.
USP PAI are a product line of impurities suitable for research and analytical purposes, which help to ensure the quality and safety of medicines.
Associated Drug Substance: Amlodipine
Therapeutic Area: Cardiovascular.
For more information about this PAI, visit here.
應用
Amlodipine Methyl Analog (USP PAI) is intended for use in analytical testing to detect, identify, and measure pharmaceutical impurities.
特點和優勢
USP PAI advance your early analytical R&D and process development. PAI can be used in the following applications:
1. Conduct analytical tests during early formulation feasibility studies.
2. Determine degradation impurities produced during stress studies.
3. Develop, validate, and transfer analytical methods.
4. Perform spiking studies during process R&D to demonstrate depletion upon recrystallization.
5. Record retention times and/or spectra
6. Determine relative response factors.
7. Identify unknown impurities that formed during ICH stability conditions.
8. Identify impurities that are present in the Reference Listed Drug
9. Test for and profile impurities not listed in drug substance and drug product monographs.
1. Conduct analytical tests during early formulation feasibility studies.
2. Determine degradation impurities produced during stress studies.
3. Develop, validate, and transfer analytical methods.
4. Perform spiking studies during process R&D to demonstrate depletion upon recrystallization.
5. Record retention times and/or spectra
6. Determine relative response factors.
7. Identify unknown impurities that formed during ICH stability conditions.
8. Identify impurities that are present in the Reference Listed Drug
9. Test for and profile impurities not listed in drug substance and drug product monographs.
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
訊號詞
Danger
危險分類
Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - STOT RE 2
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務





![Dimethyl 2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate certified reference material, TraceCERT®, Manufactured by: Sigma-Aldrich Production GmbH, Switzerland](/deepweb/assets/sigmaaldrich/product/structures/632/583/0b21b85f-09ba-46d1-9cc7-482d7a705354/640/0b21b85f-09ba-46d1-9cc7-482d7a705354.png)



