推薦產品
等級
pharmaceutical primary standard
API 家族
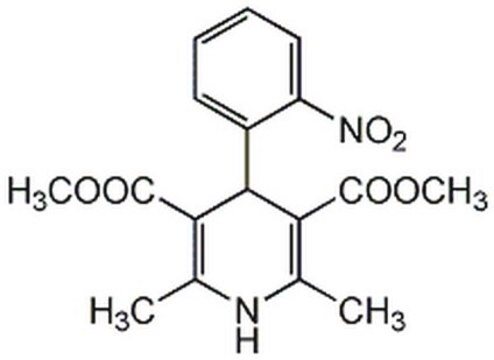
nifedipine
製造商/商標名
USP
應用
pharmaceutical (small molecule)
格式
neat
SMILES 字串
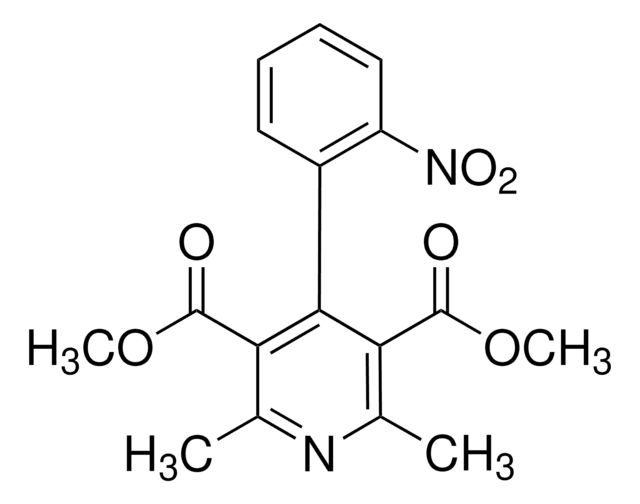
COC(=O)C1=C(C)NC(C)=C(C1c2ccccc2[N+]([O-])=O)C(=O)OC
InChI
1S/C17H18N2O6/c1-9-13(16(20)24-3)15(14(10(2)18-9)17(21)25-4)11-7-5-6-8-12(11)19(22)23/h5-8,15,18H,1-4H3
InChI 密鑰
HYIMSNHJOBLJNT-UHFFFAOYSA-N
基因資訊
human ... CACNA1C(775) , CACNA1D(776) , CACNA1F(778) , CACNA1S(779)
尋找類似的產品? 前往 產品比較指南
應用
Nifedipine USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monographs such as:
- Nifedipine Capsules
- Nifedipine Extended-Release Tablets
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
P D Henry et al.
The Journal of clinical investigation, 68(5), 1366-1369 (1981-11-01)
We tested the effects of nifedipine, a calcium antagonist, on atherogenesis in rabbits fed a 2% cholesterol diet. The drug was given orally, 40 mg/dl, and control rabbits received placebo. Nifedipine was well tolerated, and evoked only transient, moderate reductions
P D Henry
The American journal of cardiology, 46(6), 1047-1058 (1980-12-01)
Calcium antagonists (slow channel blocking agents) are a very heterogeneous group of agents with dissimilar structural, electrophysiologic and pharmacologic properties. Nifedipine is a potent, long-acting vasodilator that has proved highly efficacious in relieving anginal symptoms caused by coronary vasospasm. In
Stability studies on nifedipine tablets using thermogravimetry and differential scanning calorimetry.
Franco, P. I. B. M., E. C. Conceic?o, and M. I. G. Leles.
Journal of Thermal Analysis and Calorimetry, 93.2, 381-385 (2008)
Ryuichi Morishita et al.
Drugs, 66 Spec No 1, 31-33 (2008-01-19)
Useful drug therapy for inhibiting the extension of an aortic aneurysm or promoting its involution has not yet been established. Hypertension is a known risk factor for extension of an aortic aneurysm. However, on a cellular level it is believed
Norio Taira
Drugs, 66 Spec No 1, 1-3 (2008-01-19)
Nifedipine was synthesized by Bayer Germany in 1966 and considered for clinical use as a coronary vasodilator in patients with angina pectoris. Japanese investigators played a great part in the pre-clinical and clinical development of nifedipine. Professor Hashimoto demonstrated that
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務