推薦產品
等級
certified reference material
pharmaceutical secondary standard
品質等級
agency
traceable to BP 462
traceable to Ph. Eur. N0750000
traceable to USP 1463508
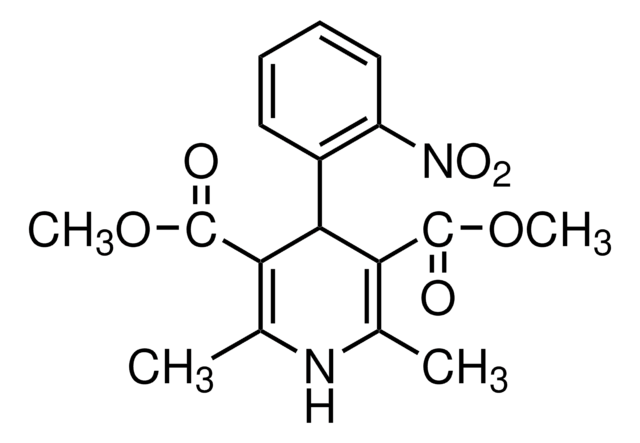
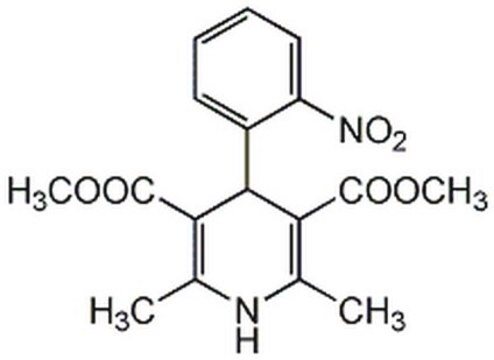
API 家族
nifedipine
CofA
current certificate can be downloaded
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-30°C
SMILES 字串
COC(=O)C1=C(C)NC(C)=C(C1c2ccccc2[N+]([O-])=O)C(=O)OC
InChI
1S/C17H18N2O6/c1-9-13(16(20)24-3)15(14(10(2)18-9)17(21)25-4)11-7-5-6-8-12(11)19(22)23/h5-8,15,18H,1-4H3
InChI 密鑰
HYIMSNHJOBLJNT-UHFFFAOYSA-N
基因資訊
human ... CACNA1C(775) , CACNA1D(776) , CACNA1F(778) , CACNA1S(779)
尋找類似的產品? 前往 產品比較指南
相關類別
一般說明
用于质量控制的药物二级标准,为制药实验室和制造商提供一种方便且性价比高的替代内部工作标准的制备方法。
硝苯地平属于钙通道拮抗剂药物,被广泛用作抗高血压药物和抗心绞痛剂。它也可以用作冠状血管扩张剂。
硝苯地平属于钙通道拮抗剂药物,被广泛用作抗高血压药物和抗心绞痛剂。它也可以用作冠状血管扩张剂。
應用
硝苯地平可用作药物参考标准品,用于通过分光光度法和色谱技术测定药物制剂和血浆样品中的分析物。
这些二级标准品是合格的认证标准物质(CRM)。它们适用于多种分析应用,包括但不限于药物释放测试、药物的定性和定量分析方法开发、食品和饮料质量控制检测以及其他校准需求。
分析報告
这些二级标准品可追溯至USP、EP(PhEur)和BP一级标准品。
其他說明
该认证标准物质(CRM)根据ISO 17034 和 ISO/IEC 17025进行生产和认证。有关此CRM使用的所有信息均可在检验报告上找到。
腳註
想要查看本品的检验报告示例,请在下框中输入LRAA0488。这只是一个示例证书,可能与您收到的批次不符。
相關產品
產品號碼
描述
訂價
客戶也查看了
Determination of nifedipine in human plasma by high-performance liquid chromatography with electrochemical detection
Suzuki H, et al.
Journal of Chromatography. B, Biomedical Sciences and Applications, 341(1), 341-347 (1985)
New spectrophotometric methods for the determination of nifedipine in pharmaceutical formulations
Rahman N and Azmi SNH
Acta Biochimica Polonica, 52(4), 915-915 (2005)
A F el Walily
Journal of pharmaceutical and biomedical analysis, 16(1), 21-30 (1998-02-03)
Three methods are described for the simultaneous determination of nifedipine and acebutolol hydrochloride in combined pharmaceutical tablets. The first method depends on first-derivative ultraviolet spectrophotometry, with peak-to-base and zero-crossing measurements methods. The first derivative amplitudes at 400 and 352 nm
Ryuichi Morishita et al.
Drugs, 66 Spec No 1, 31-33 (2008-01-19)
Useful drug therapy for inhibiting the extension of an aortic aneurysm or promoting its involution has not yet been established. Hypertension is a known risk factor for extension of an aortic aneurysm. However, on a cellular level it is believed
Norio Taira
Drugs, 66 Spec No 1, 1-3 (2008-01-19)
Nifedipine was synthesized by Bayer Germany in 1966 and considered for clinical use as a coronary vasodilator in patients with angina pectoris. Japanese investigators played a great part in the pre-clinical and clinical development of nifedipine. Professor Hashimoto demonstrated that
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務