全部照片(1)
About This Item
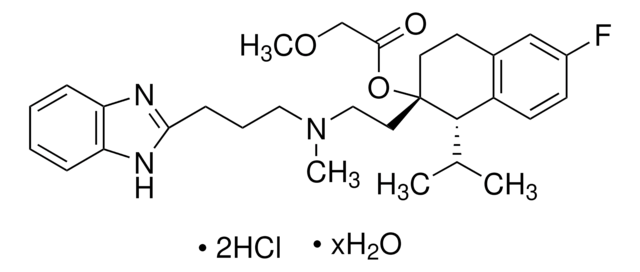
經驗公式(希爾表示法):
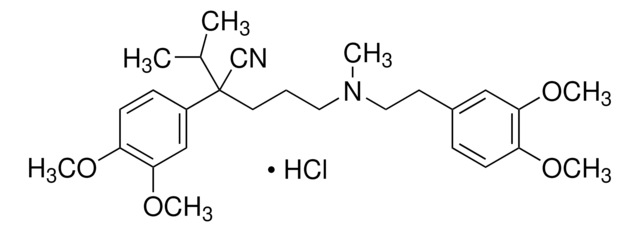
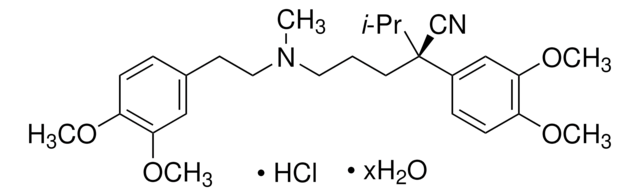
C27H38N2O4 · HCl · xH2O
CAS號碼:
分子量::
491.06 (anhydrous basis)
EC號碼:
MDL號碼:
分類程式碼代碼:
12161501
PubChem物質ID:
NACRES:
NA.77
推薦產品
品質等級
化驗
≥98% (HPLC)
形狀
powder
光學活性
[α]22/D +9.6°, c = 0.5 in ethanol(lit.)
儲存條件
desiccated
顏色
white
溶解度
H2O: >30 mg/mL
ethanol: soluble
SMILES 字串
O.Cl.COc1ccc(CCN(C)CCC[C@@](C#N)(C(C)C)c2ccc(OC)c(OC)c2)cc1OC
InChI
1S/C27H38N2O4.ClH.H2O/c1-20(2)27(19-28,22-10-12-24(31-5)26(18-22)33-7)14-8-15-29(3)16-13-21-9-11-23(30-4)25(17-21)32-6;;/h9-12,17-18,20H,8,13-16H2,1-7H3;1H;1H2/t27-;;/m1../s1
InChI 密鑰
ICKXRKHJKXMFLR-KFSCGDPASA-N
應用
R(+)-Verapamil monohydrochloride hydrate has been used as a P-glycoprotein (gp) inhibitor, to detect P-gp expression on the SK-OV-3 and SK-OV-3/DDP cell surface by flow cytometry. It has also been used as a calcium channel blocker, to evaluate its effect on doxorubicin (DOX) cytotoxicity.
生化/生理作用
Inhibitor of P-glycoprotein; less active enantiomer of (±)-verapamil.
Verapamil is a calcium channel blocker. Verapamil hydrochloride is a phenyl-alkyl amine derivative and is potentially used for treating hypertension, angina pectoris and arrhythmias. It is water soluble in nature.
注意
hygroscopic
訊號詞
Danger
危險分類
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
客戶也查看了
Effect of formulation variables on verapamil hydrochloride release from hydrated HPMC matrices
Ramrez C, et al.
Journal of the Mexican Chemical Society, 48(4), 326-331 (2004)
J A Plumb et al.
Biochemical pharmacology, 39(4), 787-792 (1990-02-15)
The L-isomer of verapamil is a more potent calcium antagonist than the D-isomer. We have examined the two stereoisomers of verapamil for their ability to increase the chemosensitivity in vitro of three drug resistant cell lines (2780AD, MCF7/AdrR and H69LX10).
E G Chikhale et al.
The Journal of pharmacology and experimental therapeutics, 273(1), 298-303 (1995-04-01)
When the blood-brain barrier (BBB) transport of a series of model peptides that varied in their physicochemical properties (lipophilicity, size and hydrogen-bonding potential) was determined using an in situ rat brain perfusion technique, an unexpected increase in flux with increasing
Assessment of the chemotherapeutic potential of a new camptothecin derivative, ZBH-1205
Wu D, et al.
Archives of Biochemistry and Biophysics, 604(1), 74-85 (2016)
Anna Blanpain et al.
ChemSusChem, 12(11), 2393-2401 (2019-04-06)
Well-controlled and extremely rapid ring-opening metathesis polymerization of unusual oxanorbornene lactam esters by Grubbs third-generation catalyst is used to prepare a range of bio-based homo- and copolymers. Bio-derived oxanorbornene lactam monomers were prepared at room temperature from maleic anhydride and
文章
Drug Transport
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務