T4580
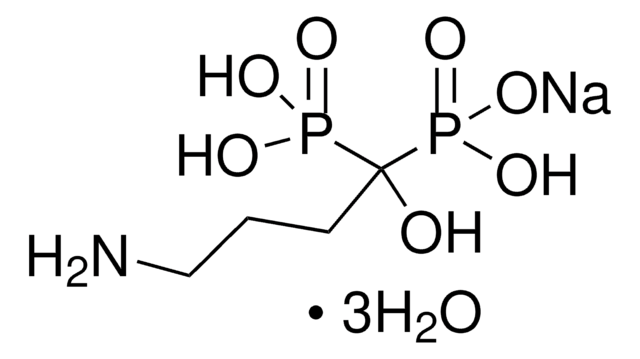
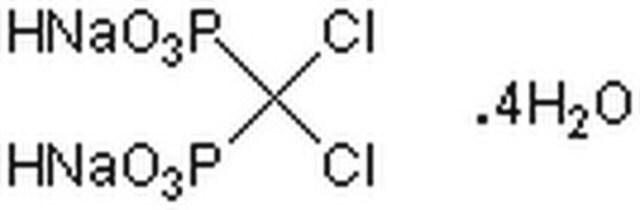
Tiludronate disodium salt hydrate
≥98% (HPLC)
同義詞:
Skelid, Tiludronic acid disodium, [[(4-Chlorophenyl)thio]methylene]bisphosphonic acid disodium salt hydrate
登入查看組織和合約定價
全部照片(1)
About This Item
經驗公式(希爾表示法):
C7H7ClNa2O6P2S · xH2O
CAS號碼:
分子量::
362.57 (anhydrous basis)
MDL號碼:
分類程式碼代碼:
12352200
PubChem物質ID:
NACRES:
NA.77
推薦產品
化驗
≥98% (HPLC)
形狀
powder
儲存條件
desiccated
顏色
white to off-white
溶解度
H2O: >10 mg/mL, clear
起源
Sanofi Aventis
SMILES 字串
O.[Na+].[Na+].OP([O-])(=O)C(Sc1ccc(Cl)cc1)P(O)([O-])=O
InChI
1S/C7H9ClO6P2S.2Na.H2O/c8-5-1-3-6(4-2-5)17-7(15(9,10)11)16(12,13)14;;;/h1-4,7H,(H2,9,10,11)(H2,12,13,14);;;1H2/q;2*+1;/p-2
InChI 密鑰
SZVJRJRMQCKFON-UHFFFAOYSA-L
生化/生理作用
Tiludronate is a bisphosphonate bone resorption inhibitor. Tiludronate inhibits protein-tyrosine-phosphatase, leading to detachment of osteoclasts from the bone surface. It also inhibits the osteoclastic proton pump and is used to treat Paget′s disease.
特點和優勢
This compound was developed by Sanofi Aventis. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
準備報告
Tiludronate disodium salt hydrate is soluble in DMSO at a concentration that is greater than or equal to 10 mg/ml.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
L Alvarez et al.
Rheumatology (Oxford, England), 43(7), 869-874 (2004-04-01)
To monitor the long-term evolution of Paget's disease activity after treatment with tiludronate by using serum total alkaline phosphatase (TAP) and more sensitive markers such as bone alkaline phosphatase (BAP), procollagen type I N propeptide (PINP) and urinary N-terminal cross-linking
Virginie Coudry et al.
American journal of veterinary research, 68(3), 329-337 (2007-03-03)
To evaluate the efficacy of tiludronate for the treatment of horses with signs of pain associated with lesions of the thoracolumbar vertebral column. 29 horses with clinical manifestations of pain associated with lesions of the thoracolumbar vertebral column and abnormal
C Delguste et al.
Journal of veterinary pharmacology and therapeutics, 31(2), 108-116 (2008-03-01)
Bioavailability and pharmacological effects of tiludronate were compared when administered as an intravenous (i.v.) bolus at a dosage of 0.1 mg/kg body weight (b.w.) once daily for 10 consecutive days (group 1, n = 6) and as a single constant
C Delguste et al.
Bone, 41(3), 414-421 (2007-07-03)
Tiludronate, a bisphosphonate, has recently been introduced in veterinary medicine to treat orthopedic conditions in the horse. This study was designed to evaluate its effects on biochemical biomarkers of bone metabolism and on bone density and structure in an experimental
Katja F Duesterdieck-Zellmer et al.
American journal of veterinary research, 73(10), 1530-1539 (2012-09-28)
To determine concentration-dependent effects of tiludronate on cartilage explants incubated with or without recombinant equine interleukin-1β (rEq IL-1). Articular cartilage explants from the femorotibial joints of 3 young adult horses. Cartilage explants were incubated with 1 of 6 concentrations (0
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務