推薦產品
生物源
non-animal source
化驗
≥98.5%
形狀
crystalline powder
製造商/商標名
Ajinomoto
技術
cell culture | mammalian: suitable
雜質
endotoxin, heavy metals, tested
mp
149-152 °C (lit.)
溶解度
H2O: 25 mg/mL
應用
pharmaceutical (small molecule)
SMILES 字串
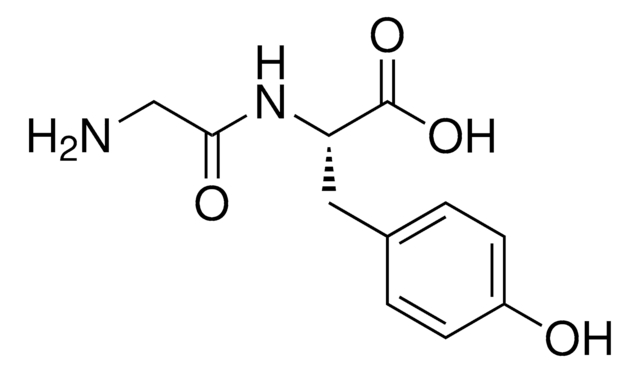
CC(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O
InChI
1S/C11H13NO4/c1-7(13)12-10(11(15)16)6-8-2-4-9(14)5-3-8/h2-5,10,14H,6H2,1H3,(H,12,13)(H,15,16)/t10-/m0/s1
InChI 密鑰
CAHKINHBCWCHCF-JTQLQIEISA-N
尋找類似的產品? 前往 產品比較指南
一般說明
To request documentation for this product, please contact Customer Support and select ‘Product Documentation′. Please note that access to documentation for this product requires a confidentiality disclosure agreement.
應用
L-Tyrosine is a non-essential amino acid. It can be used as a cell culture media component in the commercial biomanufacture of therapeutic recombinant proteins and monoclonal antibodies. N-Acetyl-L-tyrosine is an acetylated derivative of the essential amino acid L-tyrosine with reported improved nutritional properties.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
客戶也查看了
L John Hoffer et al.
JPEN. Journal of parenteral and enteral nutrition, 27(6), 419-422 (2003-11-19)
N-acetyl-L-tyrosine (NAT) is commonly used in place of tyrosine in parenteral nutrition, but human studies carried out to date indicate considerable amounts of it are excreted unchanged in the urine. NAT retention has not been well studied in parenterally fed
A R Butler et al.
Nitric oxide : biology and chemistry, 4(5), 472-482 (2000-10-06)
By the observation of chemically induced dynamic nuclear polarization in (15)N NMR spectroscopy it has been shown that nitration of N-acetyltyrosine, even under acidic conditions, is largely a radical process. In the alkaline reaction of tyrosine with peroxynitrite the main
Rakesh Kumar et al.
European journal of medicinal chemistry, 42(4), 503-510 (2006-12-26)
The development of type 2 diabetes in obese individuals is linked to lipid accumulation in non-adipose tissues. A series of N-acetyl-L-tyrosine derivatives were synthesized and evaluated for PPAR transactivation. Compounds 4d and 4f were found to show better PPARalpha transactivation
[Recording the delta-pH-generating biochemical reactions by light-addressed sensors with Ta2O5 dielectrics].
A N Reshetilov et al.
Doklady Akademii nauk, 342(5), 700-702 (1995-06-01)
M Sharma et al.
Chemico-biological interactions, 108(3), 171-185 (1998-04-07)
Dityrosine (DT) was isolated in a single-step by reversed-phase HPLC in 25% yield from enzyme-catalyzed oxidation of N-acetyl tyrosine followed by deacetylation. The isolated product was characterized by 1H NMR. A three-step chromatographic procedure was reported to facilitate the preparation
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務