推薦產品
生物源
bovine bile
synthetic
化驗
≥95%
分子量
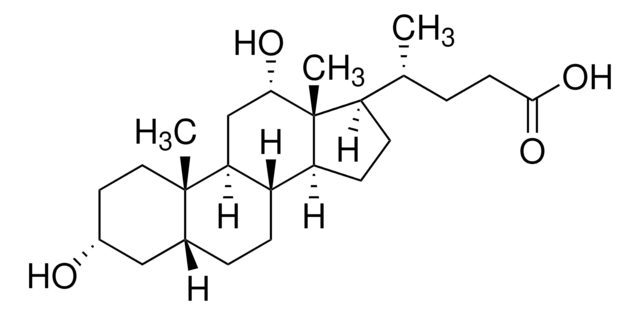
376.57 g/mol
mp
183-188 °C (lit.)
官能基
carboxylic acid
運輸包裝
ambient
儲存溫度
room temp
SMILES 字串
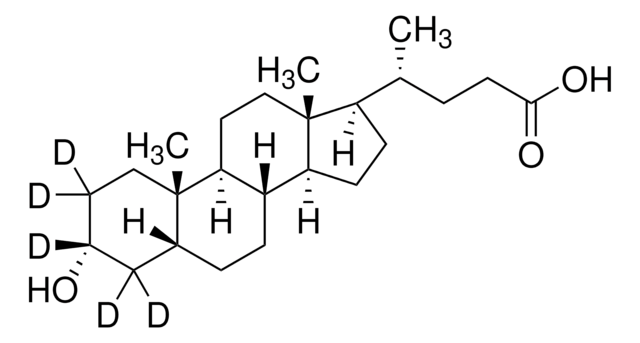
[H][C@]12CC[C@@]3([H])[C@]4([H])CC[C@H]([C@H](C)CCC(O)=O)[C@@]4(C)CC[C@]3([H])[C@@]1(C)CC[C@@H](O)C2
InChI
1S/C24H40O3/c1-15(4-9-22(26)27)19-7-8-20-18-6-5-16-14-17(25)10-12-23(16,2)21(18)11-13-24(19,20)3/h15-21,25H,4-14H2,1-3H3,(H,26,27)/t15-,16-,17-,18+,19-,20+,21+,23+,24-/m1/s1
InChI 密鑰
SMEROWZSTRWXGI-HVATVPOCSA-N
基因資訊
human ... POLA1(5422) , TOP2A(7153)
rat ... Polb(29240)
尋找類似的產品? 前往 產品比較指南
一般說明
應用
生化/生理作用
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
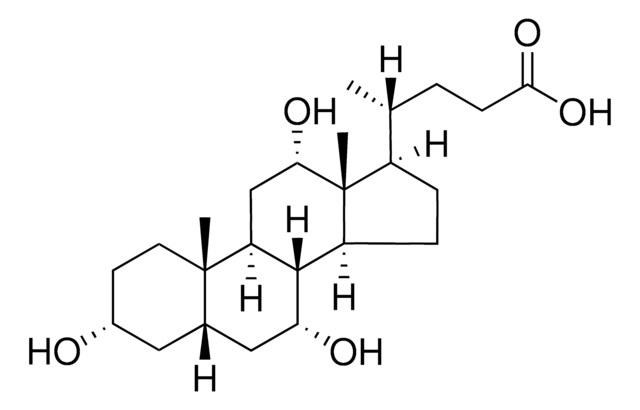
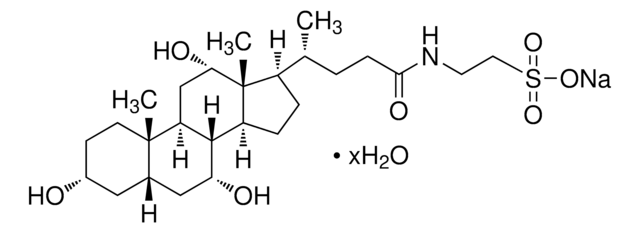
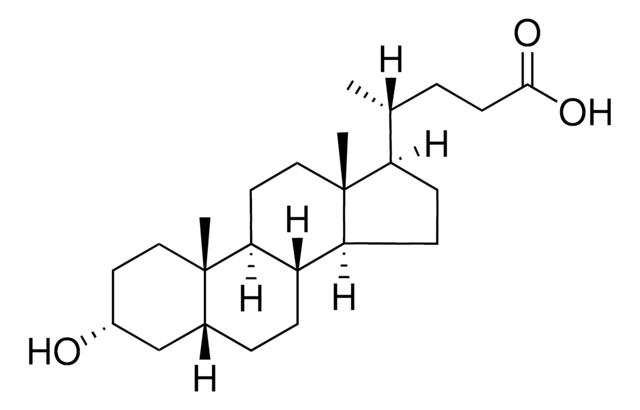
客戶也查看了
條款
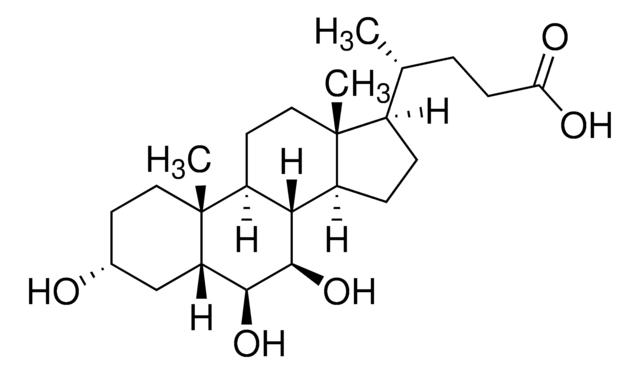
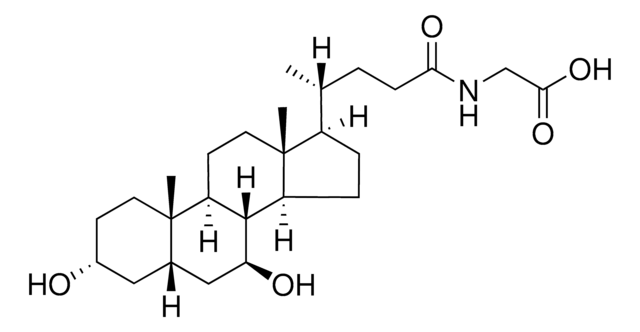
This method is particularly useful in research into the role of individual bile acids as signaling molecules; suitable for clinical laboratories to investigate potential mechanisms linked to gut hormone profiles and glycemic control.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務