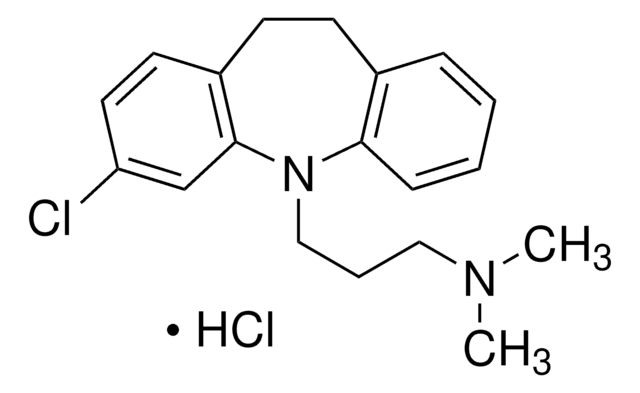
I2536
ICI 192605
≥98% (HPLC)
同義詞:
(4Z)-rel-, 4(Z)-6-(2-o-chlorophenyl-4-o-hydroxyphenyl-1,3-dioxan-cis-5-yl) hexenoic acid, 4-Hexenoic acid, 6-[(2R,4R,5S)-2-(2-chlorophenyl)-4-(2-hydroxyphenyl)-1,3-dioxan-5-yl]-
登入查看組織和合約定價
全部照片(1)
About This Item
經驗公式(希爾表示法):
C22H23ClO5
CAS號碼:
分子量::
402.87
MDL號碼:
分類程式碼代碼:
12352200
PubChem物質ID:
NACRES:
NA.77
推薦產品
化驗
≥98% (HPLC)
形狀
powder
顏色
white to tan
溶解度
DMSO: ≥20 mg/mL
起源
AstraZeneca
儲存溫度
−20°C
SMILES 字串
OC(=O)CC\C=C/C[C@H]1CO[C@H](O[C@H]1c2ccccc2O)c3ccccc3Cl
InChI
1S/C22H23ClO5/c23-18-11-6-4-9-16(18)22-27-14-15(8-2-1-3-13-20(25)26)21(28-22)17-10-5-7-12-19(17)24/h1-2,4-7,9-12,15,21-22,24H,3,8,13-14H2,(H,25,26)/b2-1-/t15-,21+,22+/m0/s1
InChI 密鑰
WHUIENZXNGAHQI-YGPRPMEGSA-N
生化/生理作用
ICI 192605 is a potent thromboxane A2 receptor antagonist. It inhibits platelet aggregation and can reverse the effects of vasoconstrictors such as TXA2 or PGD2. ICI 192605 can reverse vasoconstriction induced by inhibition of NO production by L-NEMA, which leads to an increase in TXA2 release.
特點和優勢
This compound is featured on the Prostanoid Receptors page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
This compound was developed by AstraZeneca. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
J J Descombes et al.
European journal of pharmacology, 243(2), 193-199 (1993-10-19)
The goal of the present study was to characterize the role of the endothelium in the 5-hydroxytryptamine (5-HT)-induced contraction of the rat basilar artery. Rat basilar artery segments were mounted in myographs to study their isometric tension development. 5-HT caused
Z Benyó et al.
Kidney international. Supplement, 67, S218-S220 (1998-09-15)
This study investigated the role of thromboxane A2 (TXA2) and neuronal nitric oxide (NO) synthase (nNOS)-derived NO in the maintenance of resting cerebrovascular tone. Rat basilar artery (BA) segments were mounted in myographs to study their isometric tension development. 7-Nitro
N al Jarad et al.
British journal of clinical pharmacology, 37(1), 97-100 (1994-01-01)
Many prostanoids including are prostaglandin (PG) F2 alpha and PGD2 are potent bronchoconstrictor agents. There is evidence to suggest that airway thromboxane (TP) receptor may act as a common receptor for their bronchoconstrictor actions. We tested the hypothesis that inhaled
S M Hutchinson et al.
Journal of lipid mediators and cell signalling, 15(3), 249-254 (1997-03-01)
A number of eicosanoids caused plasma membrane blebbing in hepatocytes and this could be inhibited in a dose-dependent fashion by the receptor antagonists AH6809 and ICI 192605. The pattern of effectiveness of eicosanoids interfering with canalicular vacuole accumulation in hepatocyte
Z Benyó et al.
Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism, 18(6), 616-618 (1998-06-17)
Inhibition of nitric oxide (NO) synthesis induces vasoconstriction and reduction of the blood flow in the brain, indicating that basal release of NO provides a resting vasorelaxant tone in the cerebral circulation. In the present study, the contractile effect of
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務