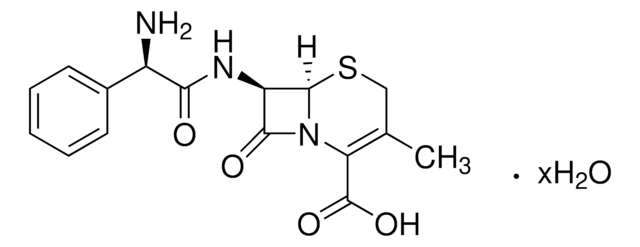
全部照片(1)
About This Item
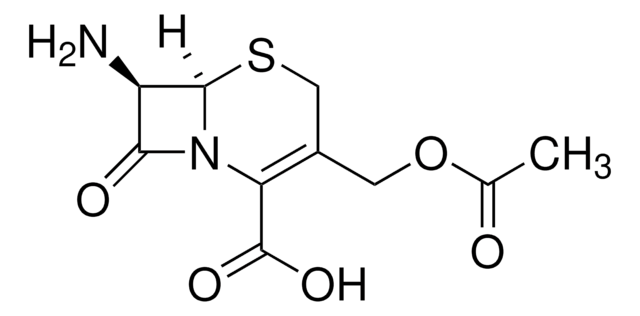
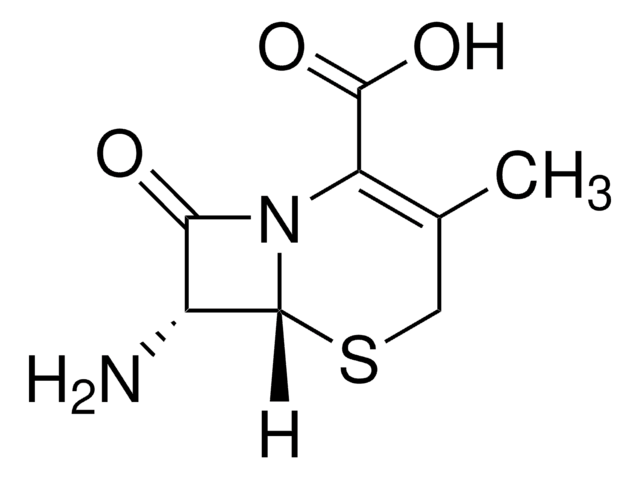
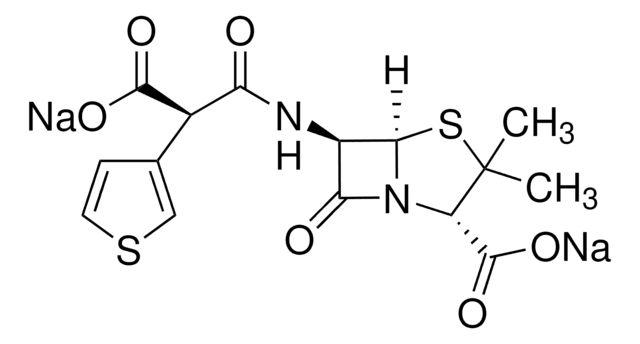
經驗公式(希爾表示法):
C8H10N2O3S
CAS號碼:
分子量::
214.24
EC號碼:
MDL號碼:
分類程式碼代碼:
51102829
PubChem物質ID:
NACRES:
NA.85
推薦產品
形狀
powder or crystals
品質等級
抗生素活性譜
Gram-positive bacteria
作用方式
cell wall synthesis | interferes
儲存溫度
2-8°C
SMILES 字串
CC1=C(N2[C@H](SC1)[C@H](N)C2=O)C(O)=O
InChI
1S/C8H10N2O3S/c1-3-2-14-7-4(9)6(11)10(7)5(3)8(12)13/h4,7H,2,9H2,1H3,(H,12,13)/t4-,7-/m1/s1
InChI 密鑰
NVIAYEIXYQCDAN-CLZZGJSISA-N
尋找類似的產品? 前往 產品比較指南
一般說明
化学结构:β-内酰胺
應用
7-氨基去乙酰氧基头孢烷酸用于头孢菌素的合成和生物转化研究。
生化/生理作用
7-ADCA是由产黄青霉菌产生的青霉素G生产的,涉及几个污染的化学步骤,然后使用青霉素酰化酶进行酶脱酰。
包裝
无底玻璃瓶。内含物装在插入的融合锥内。
其他說明
保存于密闭容器内,置于干燥通风处。
危險聲明
防範說明
危險分類
Aquatic Chronic 3
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
Mutational analysis of a key residue in the substrate specificity of a cephalosporin acylase.
Linda G. Otten, Charles F. Sio, et al.
Chembiochem, 6, 820-825 (2004)
Linda G Otten et al.
The Journal of biological chemistry, 277(44), 42121-42127 (2002-08-29)
Using directed evolution, we have selected an adipyl acylase enzyme that can be used for a one-step bioconversion of adipyl-7-aminodesacetoxycephalosporanic acid (adipyl-7-ADCA) to 7-ADCA, an important compound for the synthesis of semisynthetic cephalosporins. The starting point for the directed evolution
A N Ivankin et al.
Prikladnaia biokhimiia i mikrobiologiia, 36(3), 303-306 (2000-06-27)
The use of peptide hydrolase (EC 3.4.13.1) from Xanthomonas rubrilineans for synthesis of the antibiotic cephalexin from 7-aminodesacetoxycephalosporanic acid was studied. The optimum conditions for production of cephalexin were determined, and the yield exceeded 80%. A method for monitoring the
C G P H Schroën et al.
Biotechnology and bioengineering, 80(2), 144-155 (2002-09-05)
Integrated process concepts for enzymatic cephalexin synthesis were investigated by our group, and this article focuses on the integration of reactions and product removal during the reactions. The last step in cephalexin production is the enzymatic kinetic coupling of activated
C G Schroën et al.
Biotechnology and bioengineering, 70(6), 654-661 (2000-11-07)
One of the building blocks of cephalosporin antibiotics is 7-amino-deacetoxycephalosporanic acid (7-ADCA). It is currently produced from penicillin G using an elaborate chemical ring-expansion step followed by an enzyme-catalyzed hydrolysis. However, 7-ADCA-like components can also be produced by direct fermentation.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務