推薦產品
生物源
Bacillus licheniformis
品質等級
形狀
powder
比活性
≥65 IU/mg
顏色
white to pale buff
抗生素活性譜
Gram-positive bacteria
作用方式
cell wall synthesis | interferes
儲存溫度
2-8°C
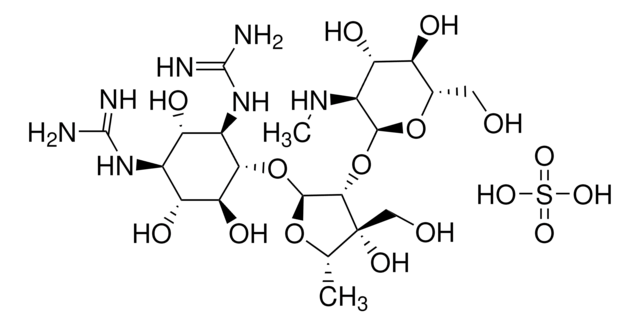
SMILES 字串
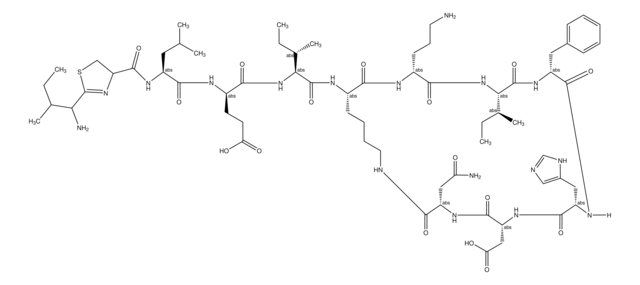
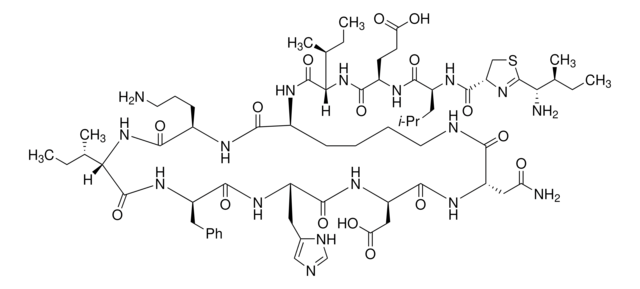
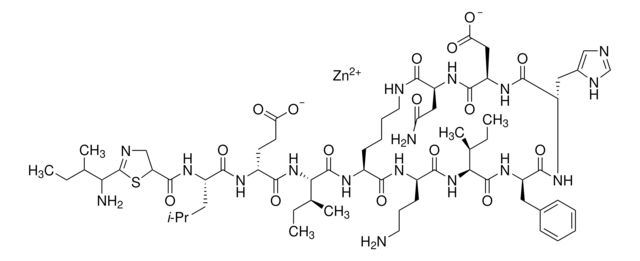
[H]N1[C@@H](Cc2cnc[nH]2)C(=O)N[C@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)NCCCC[C@H](NC(=O)[C@@H](NC(=O)[C@@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)C3CSC(=N3)C(N)C(C)CC)[C@@H](C)CC)C(=O)N[C@H](CCCN)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@H](Cc4ccccc4)C1=O
InChI
1S/C66H103N17O16S/c1-9-35(6)52(69)66-81-48(32-100-66)63(97)76-43(26-34(4)5)59(93)74-42(22-23-50(85)86)58(92)83-53(36(7)10-2)64(98)75-40-20-15-16-25-71-55(89)46(29-49(68)84)78-62(96)47(30-51(87)88)79-61(95)45(28-39-31-70-33-72-39)77-60(94)44(27-38-18-13-12-14-19-38)80-65(99)54(37(8)11-3)82-57(91)41(21-17-24-67)73-56(40)90/h12-14,18-19,31,33-37,40-48,52-54H,9-11,15-17,20-30,32,67,69H2,1-8H3,(H2,68,84)(H,70,72)(H,71,89)(H,73,90)(H,74,93)(H,75,98)(H,76,97)(H,77,94)(H,78,96)(H,79,95)(H,80,99)(H,82,91)(H,83,92)(H,85,86)(H,87,88)/t35?,36-,37-,40-,41+,42+,43-,44+,45-,46-,47+,48?,52?,53-,54-/m0/s1
InChI 密鑰
CLKOFPXJLQSYAH-RNHDWVCBSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
應用
Bacitracin has been used:
- as a protease inhibitor
- as a control antibiotic
- for the calibration in size exclusion chromatography
生化/生理作用
Antimicrobial spectrum: Gram-positive bacteria.
Mode of Action: Inhibits bacterial cell wall synthesis by inhibiting dephosphorylation of lipid pyrophosphate.
包裝
其他說明
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
客戶也查看了
文章
Inhibition of Cell Wall Biosynthesis by Antibiotics
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務