推薦產品
等級
pharmaceutical primary standard
API 家族
cyproterone
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-8°C
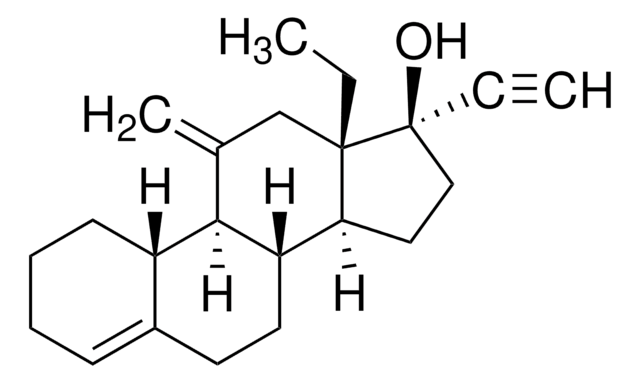
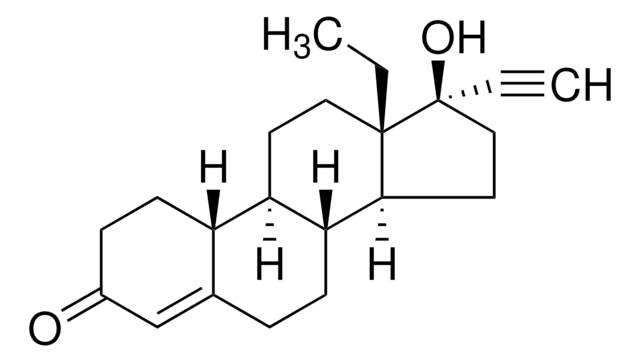
SMILES 字串
[H][C@@]12CC[C@](OC(C)=O)(C(C)=O)[C@@]1(C)CC[C@@]3([H])[C@@]2([H])C=C(Cl)C4=CC(=O)[C@@H]5C[C@@H]5[C@]34C
InChI
1S/C24H29ClO4/c1-12(26)24(29-13(2)27)8-6-16-14-10-20(25)19-11-21(28)15-9-18(15)23(19,4)17(14)5-7-22(16,24)3/h10-11,14-18H,5-9H2,1-4H3/t14-,15+,16-,17-,18-,22-,23-,24-/m0/s1
InChI 密鑰
UWFYSQMTEOIJJG-FDTZYFLXSA-N
基因資訊
human ... AR(367) , NR3C1(2908) , PGR(5241)
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Cyproterone acetate for peak identification EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
訊號詞
Warning
危險聲明
防範說明
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Carc. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
T Rabe et al.
Drug safety, 14(1), 25-38 (1996-01-01)
The preclinical safety assessment of cyproterone acetate (CPA) with regard to liver tumorigenesis was based on tumorigenicity studies, which revealed no mutagenic potential. Recently, in vitro studies on the formation of adducts and the enhancement of DNA repair synthesis with
The antiandrogen cyproterone acetate: discovery, chemistry, basic pharmacology, clinical use and tool in basic research.
F Neumann
Experimental and clinical endocrinology, 102(1), 1-32 (1994-01-01)
F Neumann et al.
Experimental and clinical endocrinology, 98(2), 71-80 (1991-01-01)
Cyproterone acetate (CPA) has been discovered more than 25 years ago and it was the first antiandrogen suitable for clinical use. CPA inhibits the action of endogenous and exogenous androgens at all androgen target organs; these include the prostate, seminal
G Friedman et al.
Digestive diseases and sciences, 44(7), 1362-1363 (1999-09-18)
Cyproterone acetate is a normally well-tolerated drug that is used widely for the treatment of prostatic carcinoma. Liver toxicity due to its use is not well known. We describe two cases of fatal fulminant hepatitis related to the use of
S L Goldenberg et al.
The Urologic clinics of North America, 18(1), 111-122 (1991-02-01)
Cyproterone acetate is a progestational antiandrogen with potent antigonadotropic activity that results in rapid suppression of serum testosterone. Used as a single agent, cyproterone acetate yields a total androgen blockade. It may be combined with low-dose diethylstilbestrol, orchiectomy, or LHRH
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務