推薦產品
等級
pharmaceutical primary standard
API 家族
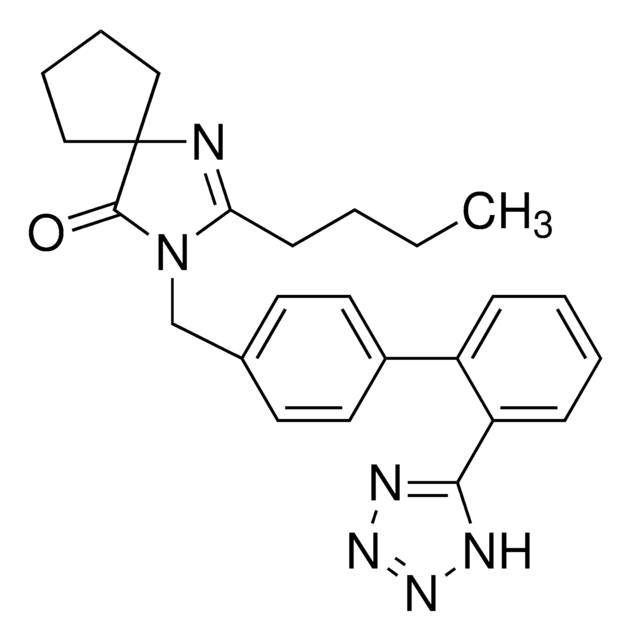
candesartan
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
SMILES 字串
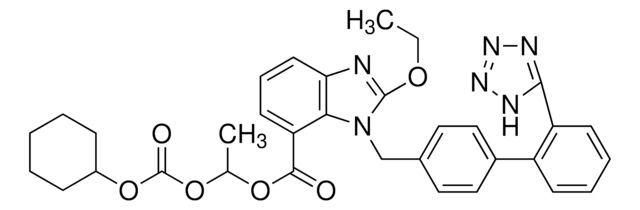
CCOc1nc2cccc(C(=O)OC(C)OC(=O)OC3CCCCC3)c2n1Cc4ccc(cc4)-c5ccccc5-c6nnn[nH]6
InChI
1S/C33H34N6O6/c1-3-42-32-34-28-15-9-14-27(31(40)43-21(2)44-33(41)45-24-10-5-4-6-11-24)29(28)39(32)20-22-16-18-23(19-17-22)25-12-7-8-13-26(25)30-35-37-38-36-30/h7-9,12-19,21,24H,3-6,10-11,20H2,1-2H3,(H,35,36,37,38)
InChI 密鑰
GHOSNRCGJFBJIB-UHFFFAOYSA-N
基因資訊
human ... AGTR1(185)
尋找類似的產品? 前往 產品比較指南
相關類別
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Candesartan cilexetil for peak identification EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生化/生理作用
坎地沙坦酯是有效的血管紧张素 II 受体拮抗剂坎地沙坦的前药形式。 该前药在肠道内被酯酶裂解释,并放活性分子。
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
訊號詞
Danger
危險聲明
危險分類
Repr. 1B - STOT RE 2 Oral
標靶器官
Kidney,Blood
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Greg L Plosker et al.
PharmacoEconomics, 24(12), 1249-1272 (2006-11-30)
The addition of candesartan cilexetil (Atacand, Amias, Blopress, Kenzen, Ratacand) to standard therapy for chronic heart failure (CHF) provided important clinical benefits at little or no additional cost in France, Germany and the UK, according to a detailed economic analysis
Gerd Bönner et al.
Current medical research and opinion, 21(6), 935-940 (2005-06-23)
Candesartan cilexetil is a highly potent and long-acting angiotensin II type I (AT1) receptor antagonist. This short review summarises results of clinical studies focusing on the duration of action of candesartan cilexetil. Results of previous clinical studies indicate that candesartan
G Mancia et al.
Journal of human hypertension, 14 Suppl 2, S3-10 (2000-11-22)
The prevention and treatment of hypertension both from the viewpoint of individual patient care and in terms of population health presents a considerable challenge to the medical profession. To assist in meeting this challenge, various bodies have produced guidelines for
Peter A Meredith
Current medical research and opinion, 23(7), 1693-1705 (2007-06-26)
Therapeutic interventions that block the renin-angiotensin-aldosterone system (RAAS) have an important role in slowing the progression of cardiovascular risk actors to established cardiovascular diseases. In recent years, angiotensin receptor blockers (ARBs) have emerged as effective and well-tolerated alternatives to an
Naoki Ichikawa et al.
Neurological research, 37(2), 147-152 (2014-08-05)
The purpose of this study was to examine whether oral administration of an angiotensin II type 1 receptor blocker (ARB) inhibited in-stent neointimal hyperplasia in carotid arteries of hypercholesterolemic rabbits. Eleven male New Zealand white rabbits were subjected to endothelial
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務