推薦產品
品質等級
化驗
≥95% (HPLC)
形狀
solid
運輸包裝
wet ice
SMILES 字串
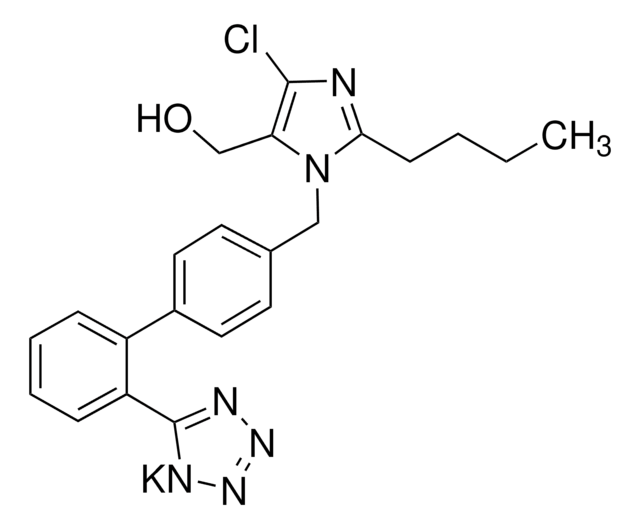
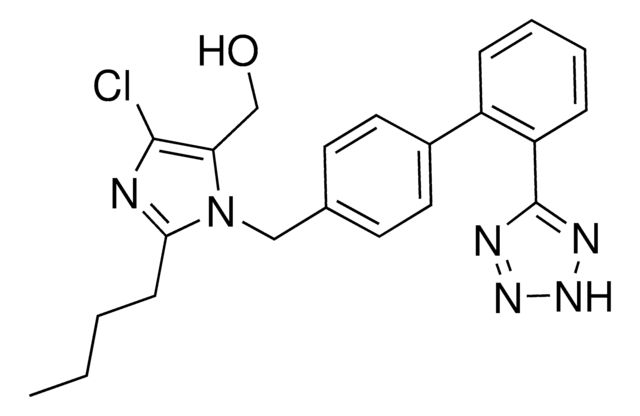
Clc1nc([n](c1C(=O)O)Cc2ccc(cc2)c3c(cccc3)c4[nH]nnn4)CCCC
InChI
1S/C22H21ClN6O2/c1-2-3-8-18-24-20(23)19(22(30)31)29(18)13-14-9-11-15(12-10-14)16-6-4-5-7-17(16)21-25-27-28-26-21/h4-7,9-12H,2-3,8,13H2,1H3,(H,30,31)(H,25,26,27,28)
InChI 密鑰
ZEUXAIYYDDCIRX-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
Losartan Carboxylic Acid (E-3174) is an active carboxylic acid metabolite of Losartan. Losartan Carboxylic Acid is a potent and selective angiotensin II receptor type 1 (AT1) antagonist.
應用
Metabolomics research
其他說明
For additional information on our range of Biochemicals, please complete this form.
訊號詞
Danger
危險聲明
危險分類
Lact. - Repr. 1B - Skin Sens. 1B
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Eleni Karatza et al.
Basic & clinical pharmacology & toxicology, 126(3), 193-202 (2019-09-13)
Losartan presents multiple peaks in the concentration-time profile. This characteristic can be attributed to gastric emptying, which is known to significantly affect the disposition of highly soluble and permeable compounds. The aim of this study was to develop a population
A Gromotowicz-Poplawska et al.
Journal of physiology and pharmacology : an official journal of the Polish Physiological Society, 70(3) (2019-10-01)
The aim of the study was to evaluate the effect of an active metabolite of losartan - EXP3174 - on a performed venous thrombus in hypertensive rat. The contribution of coagulation and fibrinolytic systems as well as platelets in the
L L Chang et al.
Journal of medicinal chemistry, 36(17), 2558-2568 (1993-08-20)
A series of 2,4-dihydro-2,4,5-trisubstituted-3H-1,2,4-triazol-3-ones was prepared via several synthetic routes and evaluated as AII receptor antagonists in vitro and in vivo. The preferred compounds contained a [2'-(5-tetrazolyl)biphenyl-4-yl]methyl side chain at N4 and an n-butyl group at C5. A number of
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務