推薦產品
等級
certified reference material
pharmaceutical secondary standard
品質等級
agency
traceable to Ph. Eur. Y0001586
traceable to USP 1044469
API 家族
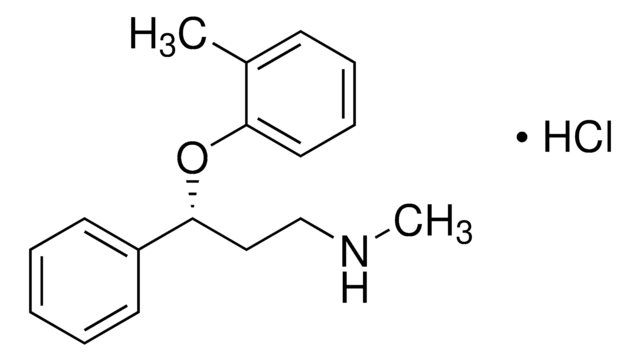
atomoxetine
CofA
current certificate can be downloaded
包裝
pkg of 500 mg
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-8°C
SMILES 字串
CNCC[C@@H](OC1=CC=CC=C1C)C2=CC=CC=C2.[H]Cl
InChI
1S/C17H21NO.ClH/c1-14-8-6-7-11-16(14)19-17(12-13-18-2)15-9-4-3-5-10-15;/h3-11,17-18H,12-13H2,1-2H3;1H/t17-;/m1./s1
InChI 密鑰
LUCXVPAZUDVVBT-UNTBIKODSA-N
基因資訊
human ... SLC6A2(6530)
尋找類似的產品? 前往 產品比較指南
一般說明
Atomoxetine hydrochloride is a nonstimulant used in the treatment of Attention-Deficit/Hyperactivity Disorder (ADHD) in children and adults.
Certified pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards
應用
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
Atomoxetine hydrochloride may be used as a pharmaceutical reference standard for the determination of atomoxetine hydrochloride in pharmaceutical formulations by spectrophotometric method.
Atomoxetine hydrochloride may be used as a pharmaceutical reference standard for the determination of atomoxetine hydrochloride in pharmaceutical formulations by spectrophotometric method.
生化/生理作用
去甲肾上腺素摄取阻滞剂。
分析報告
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
其他說明
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
腳註
To see an example of a Certificate of Analysis for this material enter LRAA6124 in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
推薦產品
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
相關產品
產品號碼
描述
訂價
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
Colourimetric assay of atomoxetine hydrochloride by simple aurum coupling reaction in bulk and tablet dosage form.
Ishaq B M, et al.
Global Journal of Medical research, 13(7B) (2014)
Joshua Caballero et al.
Clinical therapeutics, 25(12), 3065-3083 (2004-01-30)
Attention-deficit/hyperactivity disorder (ADHD) occurs in approximately 3% to 10% of the pediatric population. Most of the drugs typically used to treat ADHD are stimulants, which, because of their addictive properties and potential for abuse, are controlled substances. Although these drugs
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務