推薦產品
等級
pharmaceutical primary standard
API 家族
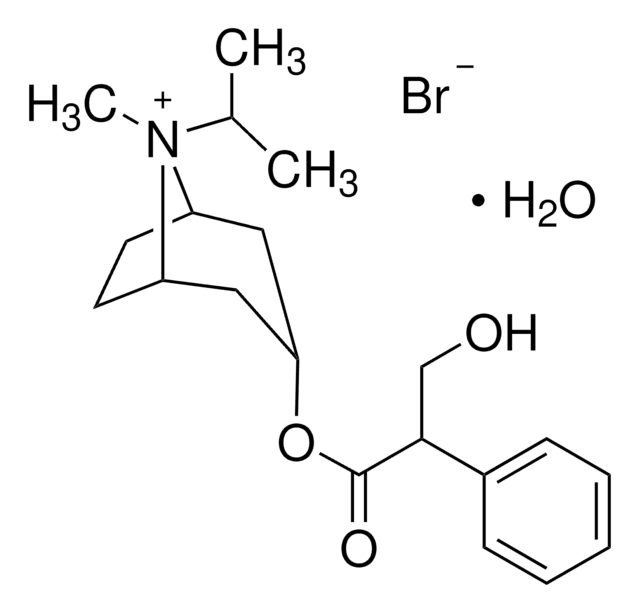
ipratropium
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
SMILES 字串
O.[Br-].CC(C)[N@+]1(C)[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c3ccccc3
InChI
1S/C20H30NO3.BrH.H2O/c1-14(2)21(3)16-9-10-17(21)12-18(11-16)24-20(23)19(13-22)15-7-5-4-6-8-15;;/h4-8,14,16-19,22H,9-13H2,1-3H3;1H;1H2/q+1;;/p-1/t16-,17+,18+,19?,21?;;
InChI 密鑰
KEWHKYJURDBRMN-XSAPEOHZSA-M
基因資訊
human ... CHRM3(1131)
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Ipratropium bromide EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
Maciej Ciebiada et al.
Allergy and asthma proceedings, 35(5), 72-79 (2014-10-09)
Persistent airways obstruction (PAO) may affect some patients with severe asthma and may significantly worsen the prognosis. This study was designed to detect risk factors associated with persistent airflow limitation in nonsmoking adult patients with severe asthma. A total of
Sharath S Hegde et al.
The Journal of pharmacology and experimental therapeutics, 351(1), 190-199 (2014-08-08)
The objective of the present studies was to characterize the pharmacologic properties of GSK-961081 [TD-5959; (R)-1-(3-((2-chloro-4-(((2-hydroxy-2-(8-hydroxy-2-oxo-1,2-dihydroquinolin-5-yl)ethyl)amino)methyl)-5-methoxyphenyl)amino)-3-oxopropyl) piperidin-4-yl [1,1'-biphenyl]-2-ylcarbamate], a novel first-in-class inhaled bifunctional compound possessing both muscarinic antagonist (MA) and β2-adrenoceptor agonist (BA) properties (MABA). In competition radioligand binding studies
Moritz Beck-Broichsitter et al.
Journal of pharmaceutical sciences, 103(8), 2585-2589 (2014-07-06)
Nebulization of active pharmaceutical ingredient (API) solutions is a well-established means to achieve pulmonary drug deposition. The current study identified the impact of formulation variables on the aerosolization performance of the eFlow(®) rapid with special respect to optimized lung application.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務