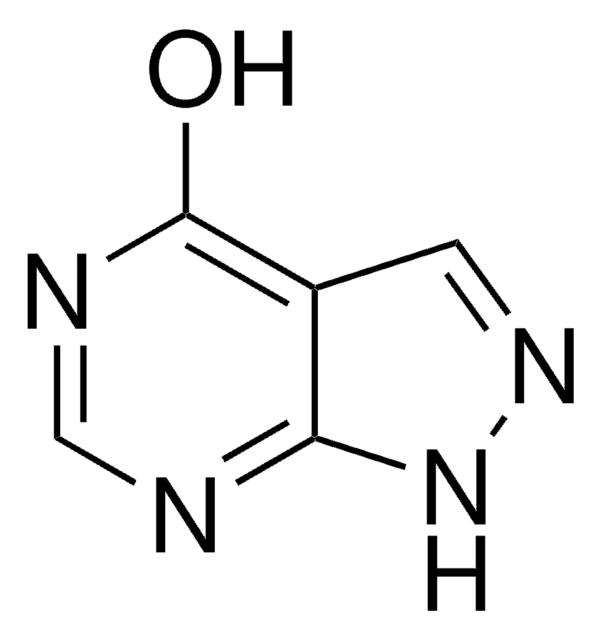
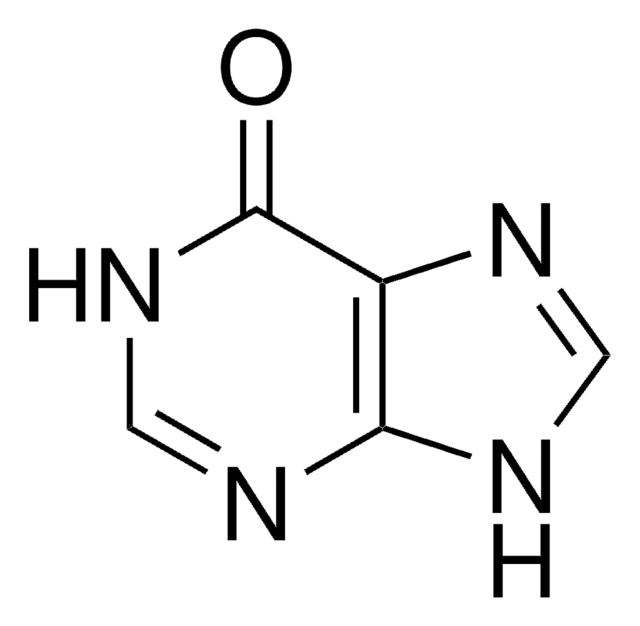
推薦產品
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. For further information and support, including product information leaflets, please go to the website of the issuing Pharmacopoeia.
應用
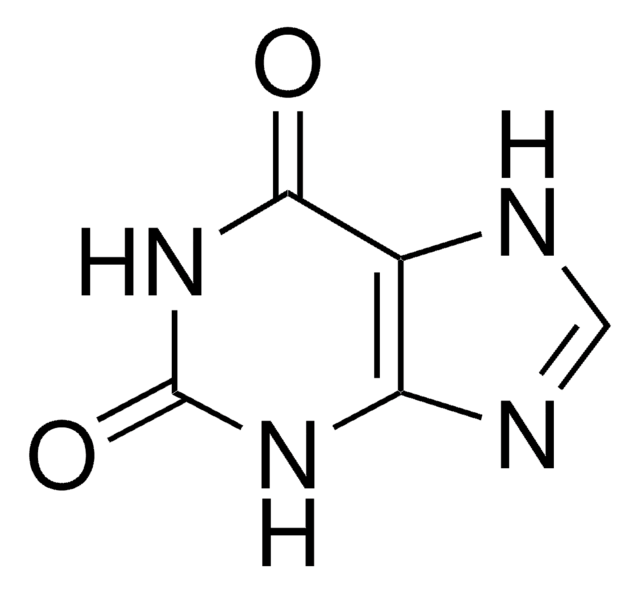
Allopurinol EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生化/生理作用
黄嘌呤氧化酶和初始嘧啶生物合成的抑制剂。治疗高尿酸血症和痛风的经典药物。
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral - Skin Sens. 1
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
Sarah Leighton et al.
Experimental dermatology, 22(3), 189-194 (2013-02-08)
Exposure to solar ultraviolet (UV) radiation suppresses adaptive immune responses. This contributes to skin carcinogenesis but may protect from some autoimmune diseases. However, the molecular changes occurring within UV-exposed skin that precipitate the downstream events leading to immune suppression are
Suborno M Ghosh et al.
Hypertension (Dallas, Tex. : 1979), 61(5), 1091-1102 (2013-04-17)
Elevation of circulating nitrite (NO2(-)) levels causes vasodilatation and lowers blood pressure in healthy volunteers. Whether these effects and the underpinning mechanisms persist in hypertension is unknown. Therefore, we investigated the consequences of systemic nitrite elevation in spontaneously hypertensive rats
Sushma Rekhraj et al.
Journal of the American College of Cardiology, 61(9), 926-932 (2013-03-02)
This study sought to ascertain if high-dose allopurinol regresses left ventricular mass (LVM) in patients with ischemic heart disease (IHD). LV hypertrophy (LVH) is common in patients with IHD including normotensive patients. Allopurinol, a xanthine oxidase inhibitor, has been shown
Essack Mitha et al.
Rheumatology (Oxford, England), 52(7), 1285-1292 (2013-03-15)
To evaluate the efficacy and safety of IL-1 inhibitor rilonacept (IL-1 Trap) for gout flare (GF) prevention during initiation of uric acid-lowering therapy (ULT) with allopurinol in a multiregional phase 3 clinical trial. Hyperuricaemic adults (n = 248) from South
Gisele Zandman-Goddard et al.
Rheumatology (Oxford, England), 52(6), 1126-1131 (2013-02-09)
To assess the adherence and persistence with allopurinol therapy among gout patients and to identify risk factors for therapy discontinuation. The study population included adults in Maccabi Healthcare Services, a 2-million member health maintenance organization in Israel, who were diagnosed
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務