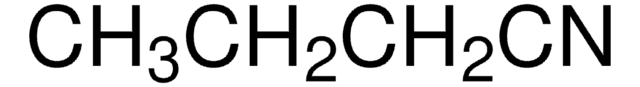推薦產品
等級
purum
品質等級
化驗
≥99.0% (GC)
形狀
liquid
折射率
n20/D 1.366 (lit.)
n20/D 1.366
bp
97 °C (lit.)
mp
−93 °C (lit.)
密度
0.772 g/mL at 25 °C (lit.)
官能基
nitrile
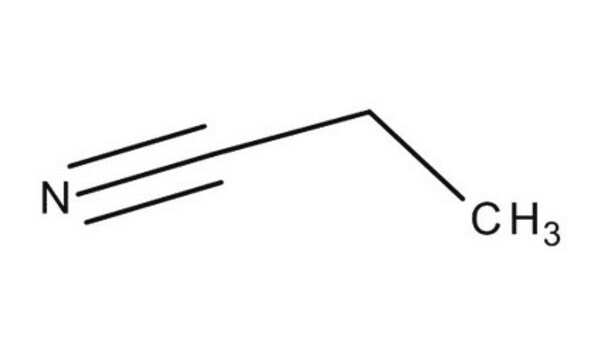
SMILES 字串
CCC#N
InChI
1S/C3H5N/c1-2-3-4/h2H2,1H3
InChI 密鑰
FVSKHRXBFJPNKK-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
- Propionitrile (PPN) is an effective solvent for catalytic asymmetric aldol reaction of a silyl enol ether with aldehydes in the presence of a chiral tin(II) Lewis acid catalyst.
- The co-solvent formed by mixing PPN with acetonitrile can be used to fabricate polymer gel electrolytes (PGEs) of dye-sensitized solar cells (DSSCs), which lead to enhanced stability of gel-state DSSCs.
- PPN can be used as a solvent for the Brønsted acid-catalyzed synthesis of N-alkyl cis-aziridines via [2+1] annulation of a diazo compound formed by the combination of an acetate and enolate. The process does not involve the use of metals or reagents and only atomic nitrogen as a co-product.
訊號詞
Danger
危險分類
Acute Tox. 2 Dermal - Acute Tox. 2 Oral - Acute Tox. 4 Inhalation - Eye Irrit. 2 - Flam. Liq. 2
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 1
閃點(°F)
42.8 °F - closed cup
閃點(°C)
6 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
Catalytic asymmetric aldol-type reaction using a chiral tin (II) Lewis acid.
Kobayashi S, et al.
Tetrahedron, 49(9), 1761-1772 (1993)
The Br?nsted acid-catalyzed direct Aza-Darzens synthesis of N-alkyl cis-aziridines.
Williams A L and Johnston J N
Journal of the American Chemical Society, 126(6), 1612-1613 (2004)
Stability improvement of gel-state dye-sensitized solar cells by utilization the co-solvent effect of propionitrile/acetonitrile and 3-methoxypropionitrile/acetonitrile with poly (acrylonitrile-co-vinyl acetate).
Venkatesan S, et al.
Journal of Power Sources, 274, 506-511 (2015)
Oliver Kaumanns et al.
The Journal of organic chemistry, 74(1), 75-81 (2008-11-27)
The rates of the reactions of the colored para-substituted phenylacetonitrile anions 1a-c and the phenylpropionitrile anions 2a-c with Michael acceptors (3a-u) were determined by UV-vis spectroscopy in DMSO at 20 degrees C. The reactions follow second-order kinetics, and the corresponding
Atsushi Kunishita et al.
Inorganic chemistry, 47(18), 8222-8232 (2008-08-14)
The copper(II) complexes 1(H) and 1(Ar(X)), supported by the N,N-di(2-pyridylmethyl)benzylamine tridentate ligand (L(H)) or its derivatives having m-substituted phenyl group at the 6-position of pyridine donor groups (L(Ar(X))), have been prepared, and their reactivity toward H2O2 has been examined in
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務