推薦產品
等級
purum
品質等級
化驗
≥98.0% (NT)
形狀
crystals
pellets
mp
108-110 °C (lit.)
111-114 °C
溶解度
methanol: 0.1 g/mL, clear, colorless to almost colorless
官能基
amine
SMILES 字串
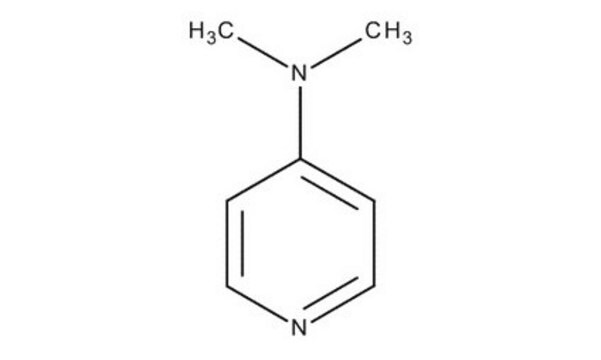
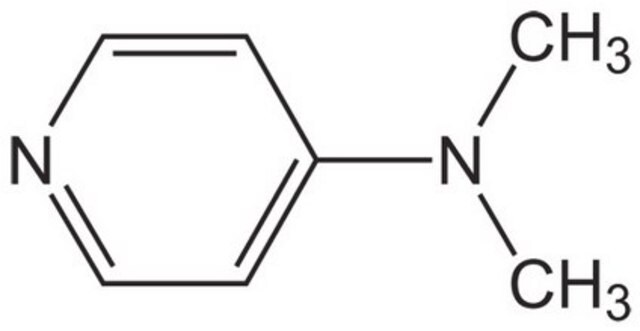
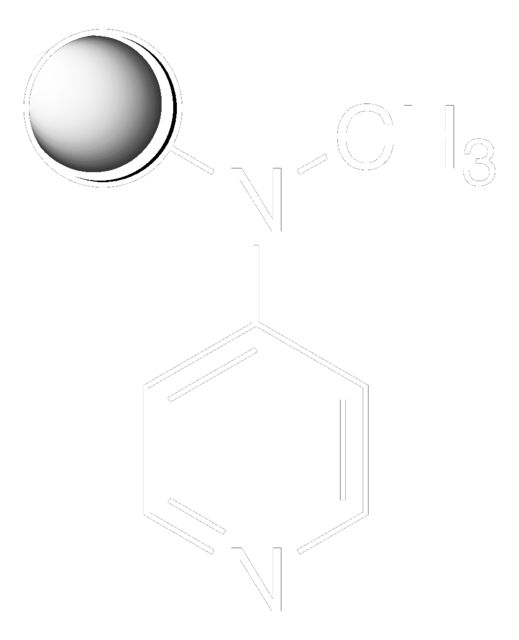
CN(C)c1ccncc1
InChI
1S/C7H10N2/c1-9(2)7-3-5-8-6-4-7/h3-6H,1-2H3
InChI 密鑰
VHYFNPMBLIVWCW-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
應用
- 作为水溶性金纳米颗粒制备中的封端剂
- 作为环氧单体聚合的引发剂
- 作为无电镀制备催化用金纳米颗粒的助剂
- 通过γ,δ-不饱和羧酸的碘内酯化制备γ-和δ-内酯的催化剂。
其他說明
訊號詞
Danger
危險分類
Acute Tox. 2 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 1
標靶器官
Nervous system
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
255.2 °F
閃點(°C)
124 °C
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
客戶也查看了
文章
In principle, the seemingly simple formation of a peptide bond can be accomplished using all the procedures available in organic chemistry for the synthesis of carboxylic acid amides. However, due to the presence of various functional groups in natural and unnatural amino acids and particularly the requirement for full retention of chiral integrity, the coupling of amino acids and peptides under mild conditions can be challenging. A plethora of coupling reagents has been developed superseding each other in efficiency and suitability for specific applications (e.g., solid-phase peptide synthesis or fragment condensation).
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務