推薦產品
蒸汽密度
3.9 (vs air)
品質等級
蒸汽壓力
0.26 mmHg ( 25 °C)
0.66 mmHg ( 38 °C)
化驗
99.6%
形狀
crystalline
自燃溫度
899 °F
expl. lim.
6.6 %
pH值
7.8 (7 g/L)
bp
200 °C (lit.)
mp
41-46 °C (lit.)
溶解度
water: soluble 135 part(lit.)
acetone: freely soluble(lit.)
alcohol: freely soluble(lit.)
carbon disulfide: freely soluble(lit.)
diethyl ether: freely soluble(lit.)
methanol: freely soluble(lit.)
oil: freely soluble(lit.)
密度
0.973 g/mL at 25 °C (lit.)
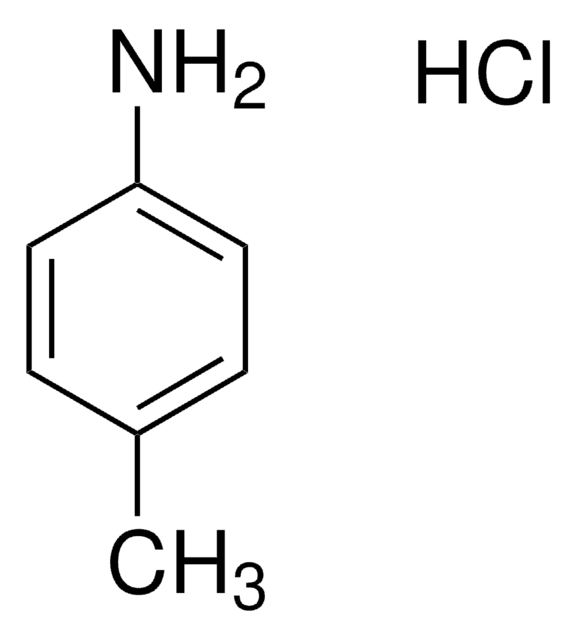
SMILES 字串
Cc1ccc(N)cc1
InChI
1S/C7H9N/c1-6-2-4-7(8)5-3-6/h2-5H,8H2,1H3
InChI 密鑰
RZXMPPFPUUCRFN-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
應用
- 芳香族偶氮化合物
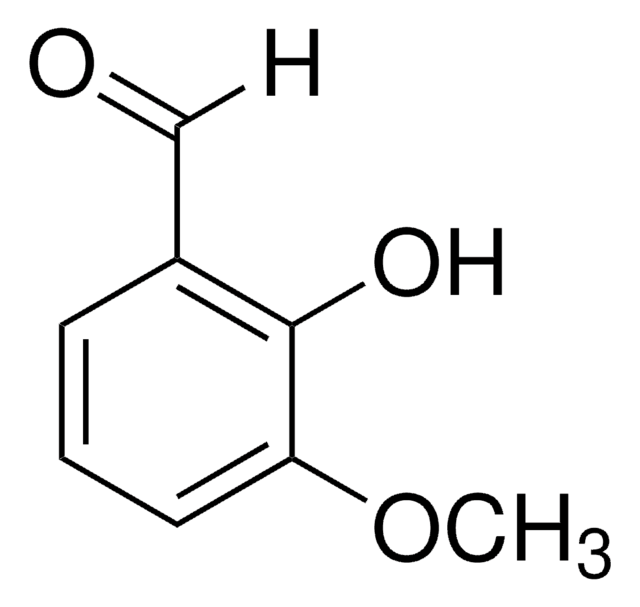
- 双齿Schiff碱配体(通过与水杨醛缩合)
訊號詞
Danger
危險分類
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 2 - Carc. 2 - Eye Irrit. 2 - Skin Sens. 1A
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
188.6 °F - closed cup
閃點(°C)
87 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
客戶也查看了
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務