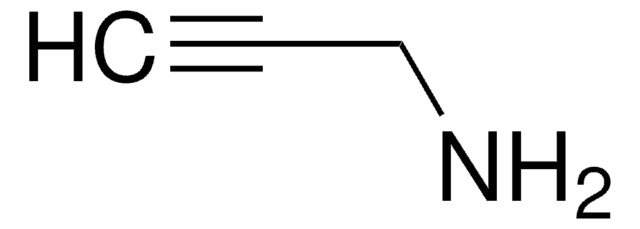
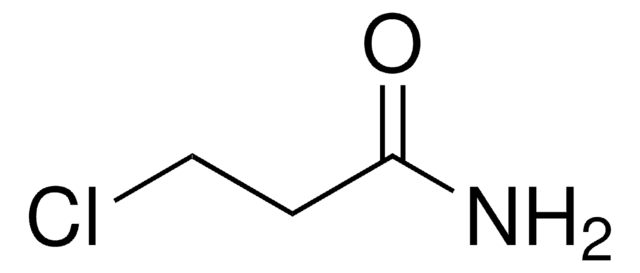
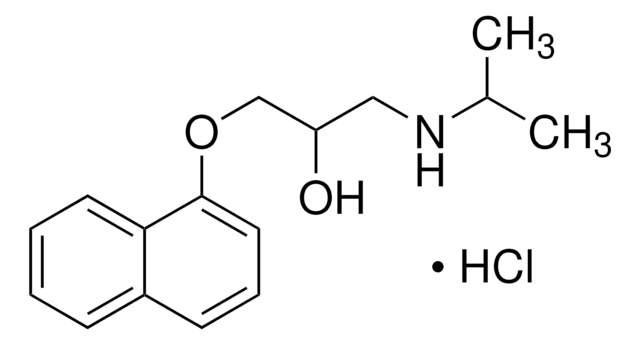
00881
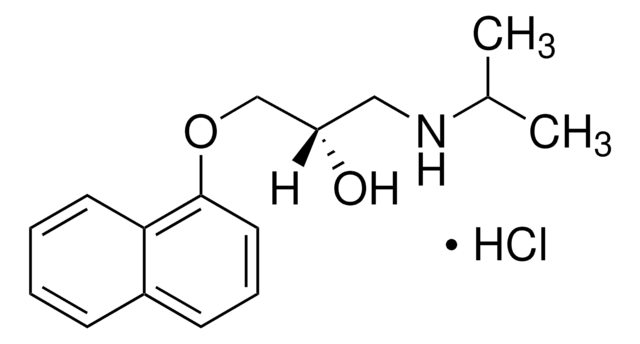
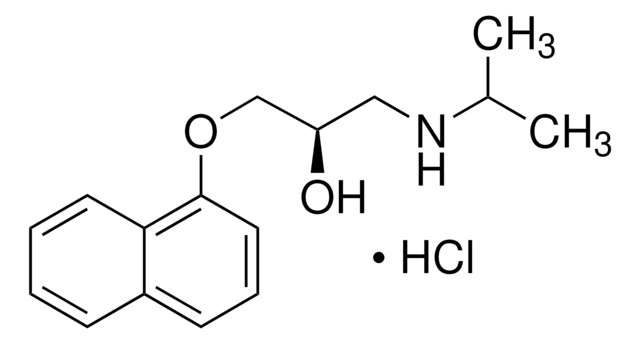
(±)-4-Hydroxypropranolol hydrochloride
analytical standard
同義詞:
1-[(4-Hydroxy-1-naphthyl)oxy]-3-(isopropylamino)- 2-propanol hydrochloride, 4′-Hydroxypropranolol hydrochloride, 4-{2-Hydroxy-3-[(1-methylethyl)amino]propoxy}-1-naphthalenol hydrochloride
登入查看組織和合約定價
全部照片(2)
About This Item
經驗公式(希爾表示法):
C16H21NO3·HCl
CAS號碼:
分子量::
311.80
Beilstein:
4036845
MDL號碼:
分類程式碼代碼:
41116107
PubChem物質ID:
NACRES:
NA.24
推薦產品
等級
analytical standard
品質等級
化驗
≥98.5% (AT)
≥98.5% (HPLC)
儲存期限
limited shelf life, expiry date on the label
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
forensics and toxicology
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-8°C
SMILES 字串
CC(C)NCC(O)COC1=CC=C(O)C2=CC=CC=C21.Cl
InChI
1S/C16H21NO3.ClH/c1-11(2)17-9-12(18)10-20-16-8-7-15(19)13-5-3-4-6-14(13)16;/h3-8,11-12,17-19H,9-10H2,1-2H3;1H
InChI 密鑰
ROUJENUXWIFONU-UHFFFAOYSA-N
推薦產品
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Takeshi Shimizudani et al.
Chemico-biological interactions, 183(1), 67-78 (2009-10-27)
Oxidative metabolism of propranolol (PL) enantiomers (R-PL and S-PL) to 4-hydroxypropranolol (4-OH-PL), 5-OH-PL and N-deisopropylpropranolol (NDP) was examined in hepatic microsomes from cynomolgus and marmoset monkeys and in small intestinal microsomes from monkeys and humans. In hepatic microsomes, levels of
Propranolol 4- and 5-hydroxylation and N-desisopropylation by cloned human cytochrome P4501A1 and P4501A2.
M S Ching et al.
Drug metabolism and disposition: the biological fate of chemicals, 24(6), 692-694 (1996-06-01)
S Narimatsu et al.
Chemical research in toxicology, 8(5), 721-728 (1995-07-01)
We have characterized a chemically reactive propranolol (PL) metabolite which binds to proteins in rat liver microsomes. During incubation with rat liver microsomes (1 mg of protein) fortified with an NADPH-generating system, 4-hydroxypropranolol (4-OH-PL) quickly disappeared from the reaction medium
Keyller Bastos Borges et al.
Electrophoresis, 30(22), 3910-3917 (2009-10-31)
A CE method is described for the enantioselective analysis of propranolol (Prop) and 4-hydroxypropranolol (4-OH-Prop) in liquid Czapek medium with application in the study of the enantioselective biotransformation of Prop by endophytic fungi. The electrophoretic conditions previously optimized were as
V L Herring et al.
Journal of chromatography, 612(2), 215-221 (1993-02-26)
A method is described for quantitation of underivatized enantiomers of propranolol and its major basic metabolite, 4-hydroxypropranolol, in urine samples by high-performance liquid chromatography with fluorescence detection, using a cellulose tris(3,5-dimethylphenylcarbamate) chiral stationary phase. This method was found to be
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務